Neurodegenerative Patterns of Cognitive Clusters of Early-Onset Alzheimer's Disease Subjects: Evidence for Disease Heterogeneity
- PMID: 31901905
- PMCID: PMC7031037
- DOI: 10.1159/000504341
Neurodegenerative Patterns of Cognitive Clusters of Early-Onset Alzheimer's Disease Subjects: Evidence for Disease Heterogeneity
Abstract
Background/aims: Alzheimer's disease (AD) with onset before 65 (early-onset AD [EOAD]) occurs in approximately 6% of cases and can affect nonmemory domains. Here, we analyze patterns of impairment in amnestic EOAD individuals using data-driven statistical analyses.
Methods: Cognitive data of 146 EOAD subjects were Z-normalized to 395 cognitively normal (CN) individuals. Domain-averaged Z-scores were adjusted for age, sex, and education followed by Wald cluster analysis of residuals. Magnetic resonance imaging and positron emission tomography comparisons of EOAD clusters to age-matched CN were done using Statistic Parametric Mapping 8. Cluster-level-family-wise error (p < 0.05) correction was applied. Mixed-effect models were used to compute longitudinal change across clusters.
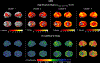
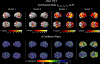
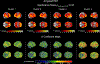
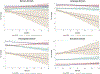
Results: Scree plot using the pseudo-T-squared suggested a 4-cluster solution. Cluster 1 (memory-predominant impairment) showed atrophy/hypometabolism in medial/lateral temporal, lateral parietal, and posterior cingulate regions. Cluster 2 (memory/visuospatial-predominant) showed atrophy/hypometabolism of medial temporal, temporoparietal, and frontal cortices. Cluster 3 (memory, language, and executive function) and Cluster 4 (globally impaired) manifested atrophy and hypometabolism throughout the brain. Longitudinally between-cluster differences in the visuospatial and language/executive domains were significant, suggesting phenotypic variation.
Conclusion: We observed significant heterogeneity in cognitive presentation among amnestic EOAD subjects and patterns of atrophy/hypometabolism in each cluster in agreement with the observed cognitive phenotype.
Keywords: Cognition; Cognitive patterns; Early-onset Alzheimer’s disease; Heterogeneity; Magnetic resonance imaging; Positron emission tomography.
© 2020 S. Karger AG, Basel.
Conflict of interest statement
Figures
Similar articles
-
Difference in imaging biomarkers of neurodegeneration between early and late-onset amnestic Alzheimer's disease.Neurobiol Aging. 2017 Jun;54:22-30. doi: 10.1016/j.neurobiolaging.2017.02.010. Epub 2017 Feb 21. Neurobiol Aging. 2017. PMID: 28314160
-
Atrophy, hypometabolism and clinical trajectories in patients with amyloid-negative Alzheimer's disease.Brain. 2016 Sep;139(Pt 9):2528-39. doi: 10.1093/brain/aww159. Epub 2016 Jun 29. Brain. 2016. PMID: 27357349 Free PMC article.
-
Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease.Brain. 2016 May;139(Pt 5):1551-67. doi: 10.1093/brain/aww027. Epub 2016 Mar 8. Brain. 2016. PMID: 26962052 Free PMC article.
-
Early-onset Alzheimer's disease: nonamnestic subtypes and type 2 AD.Arch Med Res. 2012 Nov;43(8):677-85. doi: 10.1016/j.arcmed.2012.11.009. Epub 2012 Nov 21. Arch Med Res. 2012. PMID: 23178565 Free PMC article. Review.
-
Clinical, imaging, and pathological heterogeneity of the Alzheimer's disease syndrome.Alzheimers Res Ther. 2013 Jan 9;5(1):1. doi: 10.1186/alzrt155. eCollection 2013. Alzheimers Res Ther. 2013. PMID: 23302773 Free PMC article. Review.
Cited by
-
Magnitude and kinetics of a set of neuroanatomic volume and thickness together with white matter hyperintensity is definitive of cognitive status and brain age.Transl Psychiatry. 2024 Sep 27;14(1):389. doi: 10.1038/s41398-024-03097-2. Transl Psychiatry. 2024. PMID: 39333492 Free PMC article.
-
Tau PET positivity predicts clinically relevant cognitive decline driven by Alzheimer's disease compared to comorbid cases; proof of concept in the ADNI study.Mol Psychiatry. 2025 Feb;30(2):587-599. doi: 10.1038/s41380-024-02672-9. Epub 2024 Aug 23. Mol Psychiatry. 2025. PMID: 39179903 Free PMC article.
-
"Advances in biomarker discovery and diagnostics for alzheimer's disease".Neurol Sci. 2025 Jun;46(6):2419-2436. doi: 10.1007/s10072-025-08023-y. Epub 2025 Feb 1. Neurol Sci. 2025. PMID: 39893357 Review.
-
Investigating differences in young- and late-onset progressive supranuclear palsy.J Neurol. 2023 Dec;270(12):6103-6112. doi: 10.1007/s00415-023-11976-9. Epub 2023 Sep 5. J Neurol. 2023. PMID: 37670149
-
Recent update on the heterogeneity of the Alzheimer's disease spectrum.J Neural Transm (Vienna). 2022 Jan;129(1):1-24. doi: 10.1007/s00702-021-02449-2. Epub 2021 Dec 17. J Neural Transm (Vienna). 2022. PMID: 34919190 Review.
References
-
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 2017;13(4):325–73. - PubMed
-
- Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA: Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis 2010;19:1401–1408. - PubMed
-
- Ye BS, Seo SW, Lee Y, Kim SY, Choi SH, Lee YM, Kim DH, Han HJ, Na DL, Kim EJ: Neuropsychological Performance and Conversion to Alzheimer’s Disease in Early- Compared to Late-Onset Amnestic Mild Cognitive Impairment: CREDOS Study. Dementia and Geriatric Cognitive Disorders 2012;34:156–166. - PubMed
-
- van der Flier WM, Pijnenburg YAL, Fox NC, Scheltens P: Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE epsilon 4 allele. Lancet Neurol 2011;10:280–288. - PubMed

