Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors
- PMID: 31902145
- PMCID: PMC6943255
- DOI: 10.4093/dmj.2019.0174
Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors
Abstract
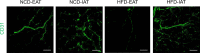
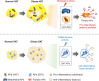
Chronic energy surplus increases body fat, leading to obesity. Since obesity is closely associated with most metabolic complications, pathophysiological roles of adipose tissue in obesity have been intensively studied. White adipose tissue is largely divided into subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). These two white adipose tissues are similar in their appearance and lipid storage functions. Nonetheless, emerging evidence has suggested that SAT and VAT have different characteristics and functional roles in metabolic regulation. It is likely that there are intrinsic differences between VAT and SAT. In diet-induced obese animal models, it has been reported that adipogenic progenitors in VAT rapidly proliferate and differentiate into adipocytes. In obesity, VAT exhibits elevated inflammatory responses, which are less prevalent in SAT. On the other hand, SAT has metabolically beneficial effects. In this review, we introduce recent studies that focus on cellular and molecular components modulating adipogenesis and immune responses in SAT and VAT. Given that these two fat depots show different functions and characteristics depending on the nutritional status, it is feasible to postulate that SAT and VAT have different developmental origins with distinct adipogenic progenitors, which would be a key determining factor for the response and accommodation to metabolic input for energy homeostasis.
Keywords: Adipogenesis; Adipose tissue; Energy metabolism; Inflammation; Obesity; Stem cells.
Copyright © 2019 Korean Diabetes Association.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


Similar articles
-
A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents.Diabetes. 2016 Mar;65(3):610-8. doi: 10.2337/db15-1478. Epub 2015 Dec 30. Diabetes. 2016. PMID: 26718495 Free PMC article.
-
Under the Surface of Subcutaneous Adipose Tissue Biology.Acta Dermatovenerol Croat. 2016 Dec;24(4):250-260. Acta Dermatovenerol Croat. 2016. PMID: 28128075 Review.
-
Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response.J Clin Endocrinol Metab. 2011 Jan;96(1):E73-82. doi: 10.1210/jc.2010-1575. Epub 2010 Nov 3. J Clin Endocrinol Metab. 2011. PMID: 21047918
-
A long-term high-fat diet induces differential gene expression changes in spatially distinct adipose tissue of male mice.Physiol Genomics. 2024 Dec 1;56(12):819-832. doi: 10.1152/physiolgenomics.00080.2024. Epub 2024 Sep 30. Physiol Genomics. 2024. PMID: 39348460 Free PMC article.
-
Dysmetabolic adipose tissue in obesity: morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects.J Endocrinol Invest. 2021 May;44(5):921-941. doi: 10.1007/s40618-020-01446-8. Epub 2020 Nov 3. J Endocrinol Invest. 2021. PMID: 33145726 Review.
Cited by
-
Obesity and the risk of cardiometabolic diseases.Nat Rev Cardiol. 2023 Jul;20(7):475-494. doi: 10.1038/s41569-023-00847-5. Epub 2023 Mar 16. Nat Rev Cardiol. 2023. PMID: 36927772 Review.
-
NOTCH1 as a Negative Regulator of Avian Adipocyte Differentiation: Implications for Fat Deposition.Animals (Basel). 2024 Feb 9;14(4):585. doi: 10.3390/ani14040585. Animals (Basel). 2024. PMID: 38396553 Free PMC article.
-
WT1 in Adipose Tissue: From Development to Adult Physiology.Front Cell Dev Biol. 2022 Mar 16;10:854120. doi: 10.3389/fcell.2022.854120. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372335 Free PMC article. Review.
-
Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet.Int J Mol Sci. 2021 Jan 19;22(2):966. doi: 10.3390/ijms22020966. Int J Mol Sci. 2021. PMID: 33478060 Free PMC article.
-
snRNA-seq reveals subcutaneous white adipose tissue remodeling upon return to thermoneutrality after cold stimulation.Front Cell Dev Biol. 2025 May 22;13:1578180. doi: 10.3389/fcell.2025.1578180. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40476003 Free PMC article.
References
-
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. - PubMed
-
- Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. - PMC - PubMed
-
- Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, Seong JK, Seok YJ, Kim JB. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015;29:2397–2411. - PubMed
-
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

