Apical periodontitis-induced mechanical allodynia: A mouse model to study infection-induced chronic pain conditions
- PMID: 31902318
- PMCID: PMC6977224
- DOI: 10.1177/1744806919900725
Apical periodontitis-induced mechanical allodynia: A mouse model to study infection-induced chronic pain conditions
Abstract
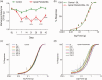
Infection-induced chronic pain is an under-studied pain condition. One example is apical periodontitis, which evokes considerable mechanical allodynia that persists after treatment in 7% to 12% of patients. Available analgesics often provide incomplete relief. However, a preclinical model to study pain mechanisms associated with apical periodontitis is not available. Here, we report a mouse model of apical periodontitis to facilitate studies determining mechanisms mediating persistent infection-induced pain. Mice were anesthetized and the left first molar was exposed to the oral environment for six weeks. Bone resorption, as an indicator of apical periodontitis, was quantified using microcomputed tomography. Mechanical allodynia was determined using extraoral von-Frey filaments in both male and female mice. The expression of c-fos in the medullary dorsal horn was assessed using immunohistochemistry. Mice with apical periodontitis developed significant mechanical allodynia by day 7 that was maintained for 42 days. Mechanical thresholds were significantly lower in females compared to males. Administration of ibuprofen, morphine, or MK-801 reversed mechanical allodynia. Finally, apical periodontitis triggered an upregulation of c-fos in the medullary dorsal horn. Collectively, this model simulates signs of clinical pain experienced by patients with apical periodontitis, detects sex differences in allodynia, and permits the study of peripheral and central trigeminal pain mechanisms.
Keywords: Apical periodontitis; central sensitization; dental pain; mechanical allodynia; orofacial pain; pain model.
Figures






Similar articles
-
Above-level mechanical hyperalgesia in rats develops after incomplete spinal cord injury but not after cord transection, and is reversed by amitriptyline, morphine and gabapentin.Pain. 2010 Oct;151(1):184-193. doi: 10.1016/j.pain.2010.07.007. Epub 2010 Aug 1. Pain. 2010. PMID: 20675054
-
Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats.Pain. 1997 May;71(1):57-64. doi: 10.1016/s0304-3959(97)03337-x. Pain. 1997. PMID: 9200174
-
The kappa opioid receptor agonist SA14867 has antinociceptive and weak sedative effects in models of acute and chronic pain.Eur J Pharmacol. 2011 Dec 5;671(1-3):53-60. doi: 10.1016/j.ejphar.2011.09.169. Epub 2011 Sep 28. Eur J Pharmacol. 2011. PMID: 21970808
-
The efficacy of morphine, pregabalin, gabapentin, and duloxetine on mechanical allodynia is different from that on neuroma pain in the rat neuropathic pain model.Anesth Analg. 2012 Jul;115(1):182-8. doi: 10.1213/ANE.0b013e31824f94ca. Epub 2012 Mar 13. Anesth Analg. 2012. PMID: 22415534
-
Spinal and supraspinal mechanisms of neuropathic pain.Ann N Y Acad Sci. 2000;909:12-24. doi: 10.1111/j.1749-6632.2000.tb06673.x. Ann N Y Acad Sci. 2000. PMID: 10911921 Review.
Cited by
-
Sex-dependent differences in the genomic profile of lingual sensory neurons in naïve and tongue-tumor bearing mice.Sci Rep. 2023 Aug 12;13(1):13117. doi: 10.1038/s41598-023-40380-6. Sci Rep. 2023. PMID: 37573456 Free PMC article.
-
Sex-specific nociceptor modulation of the apical periodontitis transcriptome.Front Mol Biosci. 2024 Feb 9;11:1338511. doi: 10.3389/fmolb.2024.1338511. eCollection 2024. Front Mol Biosci. 2024. PMID: 38404963 Free PMC article.
-
Increased Anxiety-Like Behavior in the Acute Phase of a Preclinical Model of Periodontal Disease.Front Neurol. 2020 Dec 22;11:598851. doi: 10.3389/fneur.2020.598851. eCollection 2020. Front Neurol. 2020. PMID: 33414759 Free PMC article.
-
Sex-dependent Differences in the Genomic Profile of Lingual Sensory Neurons in Naïve and Tongue-Tumor Bearing Mice.bioRxiv [Preprint]. 2023 May 14:2023.01.14.524011. doi: 10.1101/2023.01.14.524011. bioRxiv. 2023. Update in: Sci Rep. 2023 Aug 12;13(1):13117. doi: 10.1038/s41598-023-40380-6. PMID: 36711730 Free PMC article. Updated. Preprint.
References
-
- Akamine A, Hashiguchi I, Toriya Y, Maeda K. Immunohistochemical examination on the localization of macrophages and plasma cells in induced rat periapical lesions. Endod Dent Traumatol 1994; 10: 121–128. - PubMed
-
- Okiji T, Kawashima N, Kosaka T, Kobayashi C, Suda H. Distribution of Ia antigen-expressing nonlymphoid cells in various stages of induced periapical lesions in rat molars. J Endod 1994; 20: 27–31. - PubMed
-
- Falace DA, Reid K, Rayens MK. The influence of deep (odontogenic) pain intensity, quality, and duration on the incidence and characteristics of referred orofacial pain. J Orofac Pain 1996; 10: 232–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

