Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis
- PMID: 31903096
- PMCID: PMC6923693
- DOI: 10.1177/1756286419892077
Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis
Abstract
Background: With a large array of disease modifying therapies (DMTs) for relapsing-remitting MS (RRMS), identifying the optimal treatment option for the individual patient is challenging and switching of immunotherapies is often required. The objective of this study was to systematically investigate reasons for DMT switching in patients on immunotherapies for mild/moderate MS, and provide real-life insights into currently applied therapeutic strategies.
Methods: This noninterventional, cross-sectional study (ML29913) at 50 sites in Germany included RRMS patients on therapies for mild/moderate MS who switched immunotherapy in the years 2014-2017. The key outcome variable was the reason to switch, as documented in the medical charts, based on failure of current therapy, cognitive decline, adverse events (AEs), patient wish, or a woman's wish to become pregnant. Expectations of the new DMT and patients' assessment of the decision maker were also recorded.
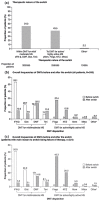
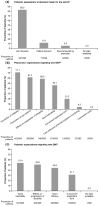
Results: The core analysis population included 595 patients, with a mean age of 41.6 years, of which 69.7% were female. More than 60% of patients had at least one relapse within 12 months prior to the switch. The main reasons to switch DMT were failure of current therapy (53.9%), patient wish (22.4%), and AEs (19.0%). Most patients (54.3%) were switched within DMTs for mild/moderate MS; only 43.5% received a subsequent DMT for active/highly active MS. While clinical and outcome-oriented aspects were the most frequently mentioned expectations of the new DMT for physicians, aspects relating to quality of life played a major role for patients.
Conclusions: Our data indicate suboptimal usage of DMTs, including monoclonal antibodies, for active/highly active MS in German patients. This illustrates the medical need for DMTs combining high efficacy, low safety risk, and low therapy burden.
Keywords: disease modifying therapies; multiple sclerosis; real-world data; relapsing-remitting multiple sclerosis; treatment switch.
© The Author(s), 2019.
Conflict of interest statement
Conflict of interest statement: All authors received support for their contribution to this study as well as medical writing support for the development of this manuscript from Roche Pharma AG, Grenzach-Wyhlen, Germany. RAL received compensation for activities with Almirall, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis Pharma, Roche and TEVA as well as research support from Biogen, Merck, and Novartis. MM received compensation for activities with Almirall, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and TEVA. EO received honoraria for studies from Abbvie, Allergan, Bayer, Biogen, Genzyme, Merck & Co., Merck Serono, Merz, Novartis, Roche, Sanofi, TEVA, UCB, and Zambon. PO received compensation for activities with Bayer, Biogen, Genzyme, Merck, Novartis, Roche, and TEVA. NR has no additional disclosures. MS received honoraria for lectures, studies and consulting from Merck, Sanofi, Novartis, and Genzyme. KTW received honoraria for lectures, studies, and consultancy from Almirall, Bayer, Biogen, Genzyme, Merck Serono, Novartis, Roche, Sanofi, and TEVA. TZ received reimbursements for participation in scientific advisory boards from Almirall, Bayer Healthcare, Biogen, Celgene, Novartis, Merck Serono, TEVA, Genzyme, and Roche; he also received speaker honoraria from Almirall, Bayer Healthcare, Biogen, Celgene, Genzyme, Novartis, Roche, TEVA, and research support from Bayer Healthcare, BAT, Biogen, Genzyme, Novartis, and TEVA. AM, SHS, JAK are current employees of Roche Pharma AG. VZ is a current employee of F. Hoffmann-La Roche.
Figures


References
-
- Burton JM, Freedman MS. The shifting landscape of disease-modifying therapies for relapsing multiple sclerosis. J Neuroophthalmol 2018; 38: 210–216. - PubMed
-
- European Multiple Sclerosis Platform. MS facts | MS treatments. http://www.emsp.org/about-ms/ms-treatments/ (accessed 23 October 2018).
-
- Grand’Maison F, Yeung M, Morrow SA, et al. Sequencing of disease-modifying therapies for relapsing-remitting multiple sclerosis: a theoretical approach to optimizing treatment. Curr Med Res Opin 2018; 34: 1419–1430. - PubMed
LinkOut - more resources
Full Text Sources

