Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion
- PMID: 31903107
- PMCID: PMC6929610
- DOI: 10.7150/thno.35841
Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion
Abstract
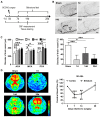
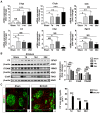
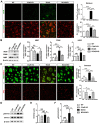
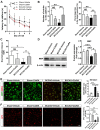
Microglial activation participates in white matter injury after cerebral hypoperfusion. However, the underlying mechanism is unclear. Here, we explore whether activated microglia aggravate white matter injury via complement C3-C3aR pathway after chronic cerebral hypoperfusion. Methods: Adult male Sprague-Dawley rats (n = 80) underwent bilateral common carotid artery occlusion for 7, 14, and 28 days. Cerebral vessel density and blood flow were examined by synchrotron radiation angiography and three-dimensional arterial spin labeling. Neurobehavioral assessments, CLARITY imaging, and immunohistochemistry were performed to evaluate activation of microglia and C3-C3aR pathway. Furthermore, C3aR knockout mice were used to establish the causal relationship of C3-C3aR signaling on microglia activation and white matter injury after hypoperfusion. Results: Cerebral vessel density and blood flow were reduced after hypoperfusion (p<0.05). Spatial learning and memory deficits and white matter injury were shown (p<0.05). These impairments were correlated with aberrant microglia activation and an increase in the number of reactive microglia adhering to and phagocytosed myelin in the hypoperfusion group (p<0.05), which were accompanied by the up-regulation of complement C3 and its receptors C3aR (p<0.05). Genetic deletion of C3ar1 significantly inhibited aberrant microglial activation and reversed white matter injury after hypoperfusion (p<0.05). Furthermore, the C3aR antagonist SB290157 decreased the number of microglia adhering to myelin (p<0.05), attenuated white matter injury and cognitive deficits in chronic hypoperfusion rats (p<0.05). Conclusions: Our results demonstrated that aberrant activated microglia aggravate white matter injury via C3-C3aR pathway during chronic hypoperfusion. These findings indicate C3aR plays a critical role in mediating neuroinflammation and white matter injury through aberrant microglia activation, which provides a novel therapeutic target for the small vessel disease and vascular dementia.
Keywords: chronic cerebral hypoperfusion; complement; inflammation; microglia; white matter injury.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC. et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–6. - PubMed
-
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R. et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

