A sericin/ graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone
- PMID: 31903148
- PMCID: PMC6929981
- DOI: 10.7150/thno.39502
A sericin/ graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone
Abstract
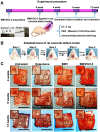
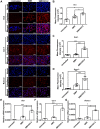
Bone defects affect millions of people worldwide each year, leading to severe disabilities. Biomimetic scaffolds mediated tissue regeneration represents a promising alternative for bone repair. However, the major problem associated with most currently clinical available artificial bone substitutes (scaffolds) is that they mainly possess filling function but lack of osteo-induction abilities. Therefore, development of biomaterials with osteo-induction property for effective bone regeneration is highly desired. Methods: We report the design and fabrication of a photo-crosslinked sericin methacryloyl (SerMA)/ graphene oxide (GO) hydrogel (SMH/GO) as a biomimetic scaffold for the functional repair of the bone. The mechanical strength, degradation and biocompatibility behavior of SMH/GO hydrogel were measured in vitro. The effect of SMH/GO hydrogel on BMSCs proliferation, migration, osteogenesis differentiation was assessed. After that, SMH/GO-2 was used as an artificial bone substitute for bone regeneration after calvarial defects and effect on bone repair was evaluated by histological, X-Ray and microCT analysis. Furthermore, the potential mechanism of SMH/GO hydrogel regulating BMSCs migration and differentiation was investigated by RNA sequencing. Results: This scaffold has good biocompatibility, cell adhesive property, proliferation- and migration-promoting effects, and osteogenic induction property. After being implanted in a rat calvarial defect model, this SMH/GO scaffold effectively promotes new bone regeneration and achieves structural and functional repair within 12 weeks by inducing autologous bone marrow-derived mesenchymal stem cells (BMSCs) differentiation. By utilizing cell-biological assays and RNA sequencing, we reveal its possible regeneration mechanisms: the SMH/GO hydrogel regulates BMSCs migration and osteo-differentiation via activating MAPK, TNF, and chemokine signaling for bone regeneration. Conclusion: Aiming to meet clinical demands and overcome current limitations of existing artificial bones, we have developed a new type of sericin/ graphene oxide composite scaffold and provided histological, functional, and molecular evidence demonstrating that it is capable of effectively repairing defective bones by inducing autologous BMSCs directional migration and osteogenic differentiation.
Keywords: Bone marrow-derived mesenchymal stem cells; Bone regeneration; Graphene oxide; Migration and osteogenesis differentiation; Sericin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







Similar articles
-
Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage.Biomaterials. 2018 May;163:89-104. doi: 10.1016/j.biomaterials.2018.02.016. Epub 2018 Feb 9. Biomaterials. 2018. PMID: 29455069
-
Off-the-Shelf Biomimetic Graphene Oxide-Collagen Hybrid Scaffolds Wrapped with Osteoinductive Extracellular Matrix for the Repair of Cranial Defects in Rats.ACS Appl Mater Interfaces. 2018 Dec 12;10(49):42948-42958. doi: 10.1021/acsami.8b11071. Epub 2018 Nov 27. ACS Appl Mater Interfaces. 2018. PMID: 30421913
-
Sericin/Nano-Hydroxyapatite Hydrogels Based on Graphene Oxide for Effective Bone Regeneration via Immunomodulation and Osteoinduction.Int J Nanomedicine. 2023 Apr 6;18:1875-1895. doi: 10.2147/IJN.S399487. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37051313 Free PMC article.
-
Tissue-specific composite hydrogel-loaded BMSCs inhibit apoptosis and promote meniscal repair.Biomater Adv. 2025 Aug;173:214258. doi: 10.1016/j.bioadv.2025.214258. Epub 2025 Mar 2. Biomater Adv. 2025. PMID: 40057992 Review.
-
[Recent advances in application of graphene oxide for bone tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 May 15;32(5):625-629. doi: 10.7507/1002-1892.201712063. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806354 Free PMC article. Review. Chinese.
Cited by
-
Combination of graphene oxide and platelet-rich plasma improves tendon-bone healing in a rabbit model of supraspinatus tendon reconstruction.Regen Biomater. 2021 Aug 4;8(6):rbab045. doi: 10.1093/rb/rbab045. eCollection 2021 Oct. Regen Biomater. 2021. PMID: 34484806 Free PMC article.
-
Fabrication and Evaluation of Silk Sericin-Derived Hydrogel for the Release of the Model Drug Berberine.Gels. 2021 Feb 20;7(1):23. doi: 10.3390/gels7010023. Gels. 2021. PMID: 33672687 Free PMC article.
-
Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine.Polymers (Basel). 2023 Jul 4;15(13):2941. doi: 10.3390/polym15132941. Polymers (Basel). 2023. PMID: 37447586 Free PMC article. Review.
-
Sericin microparticles enveloped with metal-organic networks as a pulmonary targeting delivery system for intra-tracheally treating metastatic lung cancer.Bioact Mater. 2020 Aug 22;6(1):273-284. doi: 10.1016/j.bioactmat.2020.08.006. eCollection 2021 Jan. Bioact Mater. 2020. PMID: 32913934 Free PMC article.
-
Hydrogel Drug Delivery Systems for Bone Regeneration.Pharmaceutics. 2023 Apr 25;15(5):1334. doi: 10.3390/pharmaceutics15051334. Pharmaceutics. 2023. PMID: 37242576 Free PMC article. Review.
References
-
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–9. - PubMed
-
- Kinnane N. Burden of bone disease. Eur J Oncol Nurs. 2007;11(Suppl 2):S28–31. - PubMed
-
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7. - PubMed
-
- Urist MR. Bone: Formation by Autoinduction. Science. 1965;150:893–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

