Fidelity of translation initiation is required for coordinated respiratory complex assembly
- PMID: 31903419
- PMCID: PMC6924987
- DOI: 10.1126/sciadv.aay2118
Fidelity of translation initiation is required for coordinated respiratory complex assembly
Abstract
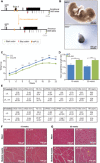
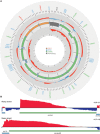
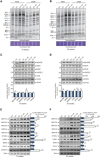
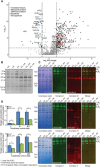
Mammalian mitochondrial ribosomes are unique molecular machines that translate 11 leaderless mRNAs; however, it is not clear how mitoribosomes initiate translation, since mitochondrial mRNAs lack untranslated regions. Mitochondrial translation initiation shares similarities with prokaryotes, such as the formation of a ternary complex of fMet-tRNAMet, mRNA and the 28S subunit, but differs in the requirements for initiation factors. Mitochondria have two initiation factors: MTIF2, which closes the decoding center and stabilizes the binding of the fMet-tRNAMet to the leaderless mRNAs, and MTIF3, whose role is not clear. We show that MTIF3 is essential for survival and that heart- and skeletal muscle-specific loss of MTIF3 causes cardiomyopathy. We identify increased but uncoordinated mitochondrial protein synthesis in mice lacking MTIF3, resulting in loss of specific respiratory complexes. Ribosome profiling shows that MTIF3 is required for recognition and regulation of translation initiation of mitochondrial mRNAs and for coordinated assembly of OXPHOS complexes in vivo.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Rackham O., Mercer T. R., Filipovska A., The human mitochondrial transcriptome and the RNA-binding proteins that regulate its expression. WIREs RNA 3, 675–695 (2012). - PubMed
-
- Greber B. J., Bieri P., Leibundgut M., Leitner A., Aebersold R., Boehringer D., Ban N., The complete structure of the 55S mammalian mitochondrial ribosome. Science 348, 303–308 (2015). - PubMed
-
- Suzuki T., Proteomic analysis of the mammalian mitochondrial ribosome. Identification of protein components in the 28 S small subunit. J. Biol. Chem. 276, 33181–33195 (2001). - PubMed
-
- Lee R. G., Rudler D. L., Rackham O., Filipovska A., Is mitochondrial gene expression coordinated or stochastic? Biochem. Soc. Trans. 46, 1239–1246 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

