Metabolic adaptation and maladaptation in adipose tissue
- PMID: 31903450
- PMCID: PMC6941795
- DOI: 10.1038/s42255-018-0021-8
Metabolic adaptation and maladaptation in adipose tissue
Abstract
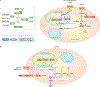
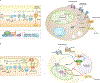
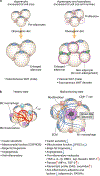
Adipose tissue possesses the remarkable capacity to control its size and function in response to a variety of internal and external cues, such as nutritional status and temperature. The regulatory circuits of fuel storage and oxidation in white adipocytes and thermogenic adipocytes (brown and beige adipocytes) play a central role in systemic energy homeostasis, whereas dysregulation of the pathways is closely associated with metabolic disorders and adipose tissue malfunction, including obesity, insulin resistance, chronic inflammation, mitochondrial dysfunction, and fibrosis. Recent studies have uncovered new regulatory elements that control the above parameters and provide new mechanistic opportunities to reprogram fat cell fate and function. In this Review, we provide an overview of the current understanding of adipocyte metabolism in physiology and disease and also discuss possible strategies to alter fuel utilization in fat cells to improve metabolic health.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

