Methotrexate plus or minus cetuximab as first-line treatment in a recurrent or metastatic (R/M) squamous cell carcinoma population of the head and neck (SCCHN), unfit for cisplatin combination treatment, a phase Ib-randomized phase II study Commence
- PMID: 31903657
- PMCID: PMC7216894
- DOI: 10.1002/hed.26053
Methotrexate plus or minus cetuximab as first-line treatment in a recurrent or metastatic (R/M) squamous cell carcinoma population of the head and neck (SCCHN), unfit for cisplatin combination treatment, a phase Ib-randomized phase II study Commence
Abstract
Background: Methotrexate in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) has limited progression-free survival (PFS) benefit. We hypothesized that adding cetuximab to methotrexate improves PFS.
Methods: In the phase-Ib-study, patients with R/M SCCHN received methotrexate and cetuximab as first-line treatment. The primary objective was feasibility. In the phase-II-study patients were randomized to this combination or methotrexate alone (2:1). The primary endpoint was PFS. Secondary endpoints were overall survival (OS), toxicity, and quality of life (QoL).
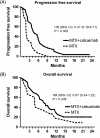
Results: In six patients in the phase-Ib-study, no dose limiting toxicities were observed. In the phase II study, 30 patients received the combination and 15 patients methotrexate. In the phase-II-study median PFS was 4.5 months in the combination group vs 2.0 months in the methotrexate group (HR 0.37; P = .002). OS, toxicity, and QoL were not significantly different.
Conclusion: Cetuximab with methotrexate improved PFS without increased toxicity in R/M SCCHN-patients.
Keywords: cetuximab; first-line; methotrexate; palliative treatment; recurrent or metastatic squamous cell carcinoma of the head and neck.
© 2020 The Authors. Head & Neck published by Wiley Periodicals, Inc.
Figures
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. - PubMed
-
- Brockstein BE. Management of recurrent head and neck cancer: recent progress and future directions. Drugs. 2011;71(12):1551‐1559. - PubMed
-
- Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116‐1127. - PubMed
-
- Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3305‐3313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical