Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk
- PMID: 31903692
- PMCID: PMC7064975
- DOI: 10.1111/dom.13955
Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk
Abstract
Aim: To investigate the effects of semaglutide versus comparators on major adverse cardiovascular events (MACE: cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) and hospitalization for heart failure (HF) in the SUSTAIN (subcutaneous semaglutide) and PIONEER (oral semaglutide) trials across subgroups of varying CV risk.
Methods: Post hoc analyses of individual patient-level data combined from SUSTAIN 6 and PIONEER 6 were performed to assess MACE and HF. MACE were analysed in subjects with and without: established CV disease and/or chronic kidney disease; prior MI or stroke; and prior HF. MACE in the SUSTAIN and PIONEER glycaemic efficacy trials were also assessed.
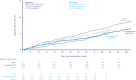
Results: In SUSTAIN 6 and PIONEER 6 combined, the hazard ratio (HR) for effect of semaglutide versus placebo on overall MACE was 0.76 (95% CI 0.62, 0.92), which was mainly driven by the effect on nonfatal stroke (HR 0.65 [95% CI 0.43, 0.97]). The HR for hospitalization for HF was 1.03 (95% CI 0.75, 1.40). The HRs for MACE were <1.0 in all subgroups, except for those with prior HF (HR 1.06 [95% CI 0.72, 1.57]); P-values for interaction of subgroup on treatment effect were >0.05, except for HF (0.046). In the combined glycaemic efficacy trials, the HR for effect of semaglutide versus comparators on MACE was 0.85 (95% CI 0.55, 1.33).
Conclusions: In SUSTAIN and PIONEER combined, glucagon-like peptide-1 analogue semaglutide showed consistent effects on MACE versus comparators across varying CV risk. No effect of semaglutide on MACE was observed in subjects with prior HF.
Trial registration: ClinicalTrials.gov NCT02054897 NCT01930188 NCT01885208 NCT02128932 NCT02305381 NCT01720446 NCT02906930 NCT02863328 NCT02607865 NCT02863419 NCT02827708 NCT02692716 NCT02849080 NCT03021187 NCT03018028 NCT03015220.
Keywords: cardiovascular disease; clinical trial; glucagon-like peptide-1 analogue; phase III study; type 2 diabetes.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
M.H. discloses personal fees for advisory panel consultancy and speaker honoraria from Boehringer Ingelheim and Janssen Inc.; grants for investigator‐initiated research studies and personal fees for advisory panel consultancy from AstraZeneca and Merck & Co; personal fees for advisory panel consultancy from Roche; and grants for investigator‐initiated preclinical research and personal fees for advisory panel consultancy and speaker honoraria from Novo Nordisk. S.C.B. discloses grants, personal fees and other potential conflicts of interest from Novo Nordisk, and personal fees and other potential conflicts of interest from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme and Sanofi‐Aventis, all for honoraria, teaching and research sponsorship or grants; also discloses other potential conflicts of interest from Elsevier and Medscape for development of educational programmes, and nonfinancial potential conflicts of interest from the All‐Wales Medicines Strategy Group, and the National Institute for Health and Care Excellence UK for providing expert advice; and discloses a potential conflict of interest for owning shares in Glycosmedia. O.K.J., R.S. and M.B.T. are full‐time employees of Novo Nordisk; O.K.J. and M.B.T. also report potential conflicts of interest for owning shares in Novo Nordisk. I.L. discloses institutional grant payments from Novo Nordisk, Novartis, Pfizer, Merck, Mylan and GI Dynamics; and personal fees from AstraZeneca, Eli Lilly, TARGETPharma, Novo Nordisk, Boehringer Ingelheim, Jenssen, Mannkind, Valeritas, Intarcia and Sanofi for consultancy. T.V. discloses personal fees from Amgen, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Bristol‐Myers Squibb, Merck Sharp & Dohme, Novo Nordisk and Sun Pharma for advisory panels and consultancy.
Figures



References
-
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. - PubMed
-
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. - PubMed
-
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. - PubMed
Publication types
MeSH terms
Substances
Associated data
- EudraCT/2013‐000632‐94
- EudraCT/2012‐004827‐19
- EudraCT/2012‐004826‐92
- EudraCT/2013‐004392‐12
- EudraCT/2013‐004502‐26
- EudraCT/2012‐002839‐28
- EudraCT/2015‐005622‐19
- EudraCT/2015‐005209‐36
- EudraCT/2015‐001351‐71
- EudraCT/2015‐005210‐30
- EudraCT/2015‐005326‐19
- EudraCT/2015‐003563‐10
- EudraCT/2015‐005593‐38
- EudraCT/2016‐000988‐16
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

