Intrinsically altered lung-resident γδT cells control lung melanoma by producing interleukin-17A in the elderly
- PMID: 31903715
- PMCID: PMC6996947
- DOI: 10.1111/acel.13099
Intrinsically altered lung-resident γδT cells control lung melanoma by producing interleukin-17A in the elderly
Abstract
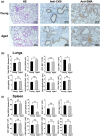
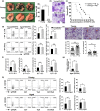
Cancer is an age-associated disease, potentially related to the altered immune system of elderly individuals. However, cancer has gradually decreased incidence in the eldest globally such as the most common lung cancer, the mechanisms of which remain to be elucidated. In this study, it was found that the number of lung-resident γδT cells was significantly increased with altered gene expression in aged mice (20-24 months) versus young mice (10-16 weeks). Aged lung Vγ4+ and Vγ6+ γδT cells predominantly produced interleukin-17A (IL-17A), resulting in increased levels in the serum and lungs. Moreover, the aged mice exhibited smaller tumors and reduced numbers of tumor foci in the lungs after challenge with intravenous injection of B16/F10 melanoma cells compared with the young mice. Aged lung Vγ4+ and Vγ6+ γδT cells were highly cytotoxic to B16/F10 melanoma cells with higher expression levels of CD103. The markedly longer survival of the challenged aged mice was dependent on γδT17 cells, since neutralization of IL-17A or depletion of indicated γδT cells significantly shortened the survival time. Consistently, supplementation of IL-17A significantly enhanced the survival time of young mice with lung melanoma. Furthermore, the anti-tumor activity of aged lung γδT17 cells was not affected by alterations in the load and composition of commensal microbiota, as demonstrated through co-housing of the aged and young mice. Intrinsically altered lung γδT17 cells underlying age-dependent changes control lung melanoma, which will help to better understand the lung cancer progression in the elderly and the potential use of γδT17 cells in anti-tumor immunotherapy.
Keywords: aging; commensal microbiota; interleukin-17A; lung cancer; lung-resident γδT cell.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared no conflict of interests.
Figures




Similar articles
-
IL-17A-Producing γδT Cells Inhibit the Formation of Malignant Pleural Effusions.Am J Respir Cell Mol Biol. 2019 Aug;61(2):174-184. doi: 10.1165/rcmb.2018-0201OC. Am J Respir Cell Mol Biol. 2019. PMID: 30608868
-
Commensal microbiota maintains alveolar macrophages with a low level of CCL24 production to generate anti-metastatic tumor activity.Sci Rep. 2017 Aug 7;7(1):7471. doi: 10.1038/s41598-017-08264-8. Sci Rep. 2017. PMID: 28785009 Free PMC article.
-
Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism.Cancer Res. 2014 Aug 1;74(15):4030-41. doi: 10.1158/0008-5472.CAN-13-2462. Epub 2014 Jun 19. Cancer Res. 2014. PMID: 24947042
-
Thymic Program Directing the Functional Development of γδT17 Cells.Front Immunol. 2018 May 8;9:981. doi: 10.3389/fimmu.2018.00981. eCollection 2018. Front Immunol. 2018. PMID: 29867959 Free PMC article. Review.
-
Research Progress of γδT Cells in Tumor Immunotherapy.Cancer Control. 2024 Jan-Dec;31:10732748241284863. doi: 10.1177/10732748241284863. Cancer Control. 2024. PMID: 39348473 Free PMC article. Review.
Cited by
-
Landscape of unconventional γδ T cell subsets in cancer.Mol Biol Rep. 2024 Jan 30;51(1):238. doi: 10.1007/s11033-024-09267-1. Mol Biol Rep. 2024. PMID: 38289417 Review.
-
Targeting Cytokine Signals to Enhance γδT Cell-Based Cancer Immunotherapy.Front Immunol. 2022 Jun 7;13:914839. doi: 10.3389/fimmu.2022.914839. eCollection 2022. Front Immunol. 2022. PMID: 35747139 Free PMC article. Review.
-
Potential of gamma/delta T cells for solid tumor immunotherapy.Front Immunol. 2024 Aug 26;15:1466266. doi: 10.3389/fimmu.2024.1466266. eCollection 2024. Front Immunol. 2024. PMID: 39253082 Free PMC article. Review.
-
The accumulation of Vγ4 T cells with aging is associated with an increased adaptive Vγ4 T cell response after foodborne Listeria monocytogenes infection of mice.Immun Ageing. 2022 May 3;19(1):19. doi: 10.1186/s12979-022-00275-y. Immun Ageing. 2022. PMID: 35501808 Free PMC article.
-
Aging unconventionally: γδ T cells, iNKT cells, and MAIT cells in aging.Semin Immunol. 2023 Sep;69:101816. doi: 10.1016/j.smim.2023.101816. Epub 2023 Aug 1. Semin Immunol. 2023. PMID: 37536148 Free PMC article. Review.
References
-
- Bourke, W. , Milstein, D. , Giura, R. , Donghi, M. , Luisetti, M. , Rubin, A. H. , & Smith, L. J. (1992). Lung cancer in young adults. Chest, 102, 1723–1729. - PubMed
-
- Bray, F. , Ferlay, J. , Soerjomataram, I. , Siegel, R. L. , Torre, L. A. , & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. - PubMed
-
- Chen, W. , Zheng, R. , Baade, P. D. , Zhang, S. , Zeng, H. , Bray, F. , … He, J. (2016a). Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians, 66(2), 115–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

