Telomere-related Markers for Cancer
- PMID: 31903880
- PMCID: PMC7475940
- DOI: 10.2174/1568026620666200106145340
Telomere-related Markers for Cancer
Abstract
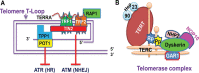
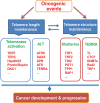
Telomeres are structurally nucleoprotein complexes at termini of linear chromosomes and essential to chromosome stability/integrity. In normal human cells, telomere length erodes progressively with each round of cell divisions, which serves as an important barrier to uncontrolled proliferation and malignant transformation. In sharp contrast, telomere maintenance is a key feature of human malignant cells and required for their infinite proliferation and maintenance of other cancer hallmarks as well. Thus, a telomere-based anti-cancer strategy has long been suggested. However, clinically efficient and specific drugs targeting cancer telomere-maintenance have still been in their infancy thus far. To achieve this goal, it is highly necessary to elucidate how exactly cancer cells maintain functional telomeres. In the last two decades, numerous studies have provided profound mechanistic insights, and the identified mechanisms include the aberrant activation of telomerase or the alternative lengthening of telomere pathway responsible for telomere elongation, dysregulation and mutation of telomereassociated factors, and other telomere homeostasis-related signaling nodes. In the present review, these various strategies employed by malignant cells to regulate their telomere length, structure and function have been summarized, and potential implications of these findings in the rational development of telomere- based cancer therapy and other clinical applications for precision oncology have been discussed.
Keywords: Cancer therapy; Gene transcription; TERC; TERRA; TERT; TERT promoter mutation; Telomerase; Telomere..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures

Similar articles
-
Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players.Oncogene. 2019 Aug;38(34):6172-6183. doi: 10.1038/s41388-019-0872-9. Epub 2019 Jul 8. Oncogene. 2019. PMID: 31285550 Free PMC article. Review.
-
Crossroads of telomere biology and anticancer drug discovery.Cancer Sci. 2020 Sep;111(9):3089-3099. doi: 10.1111/cas.14540. Epub 2020 Jul 6. Cancer Sci. 2020. PMID: 32579791 Free PMC article. Review.
-
Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies.Genome Med. 2016 Jun 20;8(1):69. doi: 10.1186/s13073-016-0324-x. Genome Med. 2016. PMID: 27323951 Free PMC article. Review.
-
Diagnosis and treatment of ALT tumors: is Trabectedin a new therapeutic option?J Exp Clin Cancer Res. 2017 Dec 22;36(1):189. doi: 10.1186/s13046-017-0657-3. J Exp Clin Cancer Res. 2017. PMID: 29273061 Free PMC article. Review.
-
Potential Telomere-Related Pharmacological Targets.Curr Top Med Chem. 2020;20(6):458-484. doi: 10.2174/1568026620666200109114339. Curr Top Med Chem. 2020. PMID: 31916516 Review.
Cited by
-
Understanding, diagnosing, and treating pancreatic cancer from the perspective of telomeres and telomerase.Cancer Gene Ther. 2024 Sep;31(9):1292-1305. doi: 10.1038/s41417-024-00768-6. Epub 2024 Apr 9. Cancer Gene Ther. 2024. PMID: 38594465 Free PMC article. Review.
-
A Novel Telomere Maintenance Gene-Related Model for Prognosis Prediction in Gastric Cancer.Biochem Genet. 2025 May 20. doi: 10.1007/s10528-025-11132-0. Online ahead of print. Biochem Genet. 2025. PMID: 40392448
-
Undersized telomeres in regulatory T cells link to the pathogenesis of allergic rhinitis.iScience. 2023 Dec 10;27(1):108615. doi: 10.1016/j.isci.2023.108615. eCollection 2024 Jan 19. iScience. 2023. PMID: 38205251 Free PMC article.
-
PITX1 plays essential functions in cancer.Front Oncol. 2023 Sep 29;13:1253238. doi: 10.3389/fonc.2023.1253238. eCollection 2023. Front Oncol. 2023. PMID: 37841446 Free PMC article. Review.
-
The telomerase gene polymorphisms, but not telomere length, increase susceptibility to primary glomerulonephritis/end stage renal diseases in females.J Transl Med. 2020 May 4;18(1):184. doi: 10.1186/s12967-020-02347-3. J Transl Med. 2020. PMID: 32366311 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

