The novel testicular enrichment protein Cfap58 is required for Notch-associated ciliogenesis
- PMID: 31904090
- PMCID: PMC6970087
- DOI: 10.1042/BSR20192666
The novel testicular enrichment protein Cfap58 is required for Notch-associated ciliogenesis
Abstract
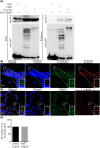
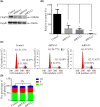
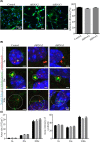
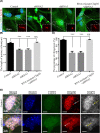
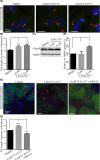
Cilia and flagella are critical organelles with conserved internal structures and diverse developmental and physiological processes according to cell type. Although the core components of structures are shared with thousands of associated proteins involved in cilia or flagella formation, we hypothesized that some unknown proteins, such as outer dense fiber 2 (Odf2/Cenexin) perform distinct functions in these organelles. In the present study, we identified several uncharacterized proteins through mass spectrometry interactome analysis of Odf2/Cenexin proteins. We further examined the expression patterns and functions of a protein named cilia and flagella associated protein 58 (Cfap58) in cultured astrocytes and sperm flagella. The results of a combination of biochemical analyses and drug administration studies reveal that Cfap58 is a testis-enrichment protein that exhibits similar localization to Odf2/Cenexin proteins and is required for the elongation of the primary cilium and sperm midpiece via modulation of the Notch signaling pathway. However, the cell cycle-related functions and localization of Odf2/Cenexin in the mother centriole were not altered in Cfap58 knockdown cells. These findings indicate that Cfap58 may be partially recruited by Odf2/Cenexin proteins and is indispensable for the cilia and flagellar assembly. These data provide us with a better understanding of ciliogenesis and flagellar elongation and may aid in identifying new targets for diseases caused by Notch-mediated ciliopathies and flagellar abnormalities.
Keywords: centrosomes; cilia; notch signalling pathway.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

