Self-assembled biomimetic Nano-Matrix for stem cell anchorage
- PMID: 31904174
- PMCID: PMC7207220
- DOI: 10.1002/jbm.a.36875
Self-assembled biomimetic Nano-Matrix for stem cell anchorage
Erratum in
-
Corrigendum: Self-assembled biomimetic Nano-Matrix for stem cell anchorage.J Biomed Mater Res A. 2021 Feb;109(2):265. doi: 10.1002/jbm.a.37096. Epub 2020 Oct 6. J Biomed Mater Res A. 2021. PMID: 33283452 No abstract available.
Abstract
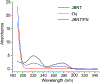
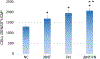
Mesenchymal stem cells (MSCs) have been widely applied in biomedicine due to their ability to differentiate into many different cell types and their ability to synthesize a broad spectrum of growth factors and cytokines that directly and indirectly influence other cells in their vicinity. To guide MSC infiltration to a bone fracture site, we developed a novel self-assembled Nano-Matrix which can be used as an injectable scaffold to repair bone fractures. The Nano-Matrix is formed by Janus base nanotubes (JBNTs) and fibronectin (FN). JBNTs are nucleobase-derived nanotubes mimicking collagen fibers, and FN is one of the cell adhesive glycoproteins which is responsible for cell-extracellular matrix interactions and guides stem cell migration and differentiation to desired cells types. Here, we demonstrated the successful fabrication and characterization of the JBNT/FN Nano-Matrix as well as its excellent bioactivity that encouraged human MSC migration and adhesion. This work lays a solid foundation for using the Nano-Matrix as an injectable approach to improve MSC retention and function during bone fracture healing.
Keywords: Janus-based nanotubes; Nano-Matrix; anchorage; fibronectin; mesenchymal stem cells.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
Y.C. is a cofounder of NanoDe Therapeutics, Inc.
Figures






Similar articles
-
Controlled Self-Assembly of DNA-Mimicking Nanotubes to Form a Layer-by-Layer Scaffold for Homeostatic Tissue Constructs.ACS Appl Mater Interfaces. 2021 Nov 3;13(43):51321-51332. doi: 10.1021/acsami.1c13345. Epub 2021 Oct 19. ACS Appl Mater Interfaces. 2021. PMID: 34663065 Free PMC article.
-
Fabrication of a Biomimetic Nano-Matrix with Janus Base Nanotubes and Fibronectin for Stem Cell Adhesion.J Vis Exp. 2020 May 10;(159):10.3791/61317. doi: 10.3791/61317. J Vis Exp. 2020. PMID: 32449715 Free PMC article.
-
Autocrine fibronectin from differentiating mesenchymal stem cells induces the neurite elongation in vitro and promotes nerve fiber regeneration in transected spinal cord injury.J Biomed Mater Res A. 2016 Aug;104(8):1902-11. doi: 10.1002/jbm.a.35720. Epub 2016 Apr 4. J Biomed Mater Res A. 2016. PMID: 26991461 Free PMC article.
-
Toward biomimetic materials in bone regeneration: functional behavior of mesenchymal stem cells on a broad spectrum of extracellular matrix components.J Biomed Mater Res A. 2010 Dec 15;95(4):1114-24. doi: 10.1002/jbm.a.32909. Epub 2010 Sep 28. J Biomed Mater Res A. 2010. PMID: 20878902
-
3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells.Int J Mol Sci. 2018 Oct 13;19(10):3150. doi: 10.3390/ijms19103150. Int J Mol Sci. 2018. PMID: 30322134 Free PMC article. Review.
Cited by
-
Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications.Int J Mol Sci. 2022 Feb 27;23(5):2640. doi: 10.3390/ijms23052640. Int J Mol Sci. 2022. PMID: 35269782 Free PMC article. Review.
-
Biosensor integrated tissue chips and their applications on Earth and in space.Biosens Bioelectron. 2023 Feb 15;222:114820. doi: 10.1016/j.bios.2022.114820. Epub 2022 Oct 20. Biosens Bioelectron. 2023. PMID: 36527831 Free PMC article. Review.
-
Controlled Self-Assembly of DNA-Mimicking Nanotubes to Form a Layer-by-Layer Scaffold for Homeostatic Tissue Constructs.ACS Appl Mater Interfaces. 2021 Nov 3;13(43):51321-51332. doi: 10.1021/acsami.1c13345. Epub 2021 Oct 19. ACS Appl Mater Interfaces. 2021. PMID: 34663065 Free PMC article.
-
Nanomaterials for Protein Delivery in Anticancer Applications.Pharmaceutics. 2021 Jan 25;13(2):155. doi: 10.3390/pharmaceutics13020155. Pharmaceutics. 2021. PMID: 33503889 Free PMC article. Review.
-
Biomaterial Drug Delivery Systems for Prominent Ocular Diseases.Pharmaceutics. 2023 Jul 15;15(7):1959. doi: 10.3390/pharmaceutics15071959. Pharmaceutics. 2023. PMID: 37514145 Free PMC article. Review.
References
-
- Aplin AE, Howe A, Alahari SK, & Juliano RL (1998). Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacological Reviews, 50(2), 197–263. - PubMed
-
- Arnold M, Cavalcanti-Adam EA, Glass R, Blummel J, Eck W, Kantlehner M, … Spatz JP (2004). Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem, 5(3), 383–388. - PubMed
-
- Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, & Feijen J (2006). Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials, 27(5), 724–734. - PubMed
-
- Chen Q; Yu HC; Chen YP (2017). U.S. Patent No. 20170362238A1, Rhode Island Hospital. https://patents.google.com/patent/US20170362238A1/en?inventor=yupeng+che....
-
- Chen Y, Bilgen B, Pareta RA, Myles AJ, Fenniri H, Ciombor DM, … Webster TJ (2010). Self-assembled rosette nanotube/hydrogel composites for cartilage tissue engineering. Tissue Engineering. Part C: Methods, 16(6), 1233–1243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

