The molecular profile of synovial fluid changes upon joint distraction and is associated with clinical response in knee osteoarthritis
- PMID: 31904489
- PMCID: PMC7054834
- DOI: 10.1016/j.joca.2019.12.005
The molecular profile of synovial fluid changes upon joint distraction and is associated with clinical response in knee osteoarthritis
Abstract
Objective: Surgical knee joint distraction (KJD) leads to clinical improvement in knee osteoarthritis (OA) and also apparent cartilage regeneration by magnetic resonance imaging. We investigated if alteration of the joint's mechanical environment during the 6 week period of KJD was associated with a molecular response in synovial fluid, and if any change was associated with clinical response.
Method: 20 individuals undergoing KJD for symptomatic radiographic knee OA had SF sampled at baseline, midpoint and endpoint of distraction (6 weeks). SF supernatants were measured by immunoassay for 10 predefined mechanosensitive molecules identified in our previous pre-clinical studies. The composite Knee injury and OA Outcome Score-4 (KOOS4) was collected at baseline, 3, 6 and 12 months.
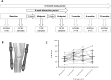
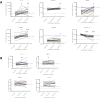
Results: 13/20 (65%) were male with mean age 54°±°5yrs. All had Kellgren-Lawrence grade ≥2 knee OA. 6/10 analytes showed statistically significant change in SF over the 6 weeks distraction (activin A; TGFβ-1; MCP-1; IL-6; FGF-2; LTBP2), P < 0.05. Of these, all but activin A increased. Those achieving the minimum clinically important difference of 10 points for KOOS4 over 6 months showed greater increases in FGF-2 and TGFβ-1 than non-responders. An increase in IL-8 during the 6 weeks of KJD was associated with significantly greater improvement in KOOS4 over 12 months.
Conclusion: Detectable, significant molecular changes are observed in SF following KJD, that are remarkably consistent between individuals. Preliminary findings appear to suggest that increases in some molecules are associated with clinically meaningful responses. Joint distraction may provide a potential opportunity in the future to define regenerative biomarker(s) and identify pathways that drive intrinsic cartilage repair.
Keywords: Biomarker; Cytokines; Distraction; Orthopaedic; Osteoarthritis; Synovial fluid.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. - PubMed
-
- Brandt K.D., Dieppe P., Radin E.L. Commentary: is it useful to subset "primary" osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum. 2009;39(2):81–95. - PubMed
-
- Burleigh A., Chanalaris A., Gardiner M.D., Driscoll C., Boruc O., Saklatvala J. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64(7):2278–2288. - PubMed
-
- Benichou C., Wirotius J.M. Articular cartilage atrophy in lower limb amputees. Arthritis Rheum. 1982;25(1):80–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

