LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis
- PMID: 31904537
- PMCID: PMC6990876
- DOI: 10.1016/j.bone.2019.115220
LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis
Abstract
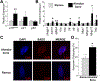
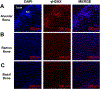
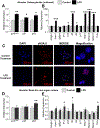
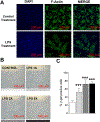
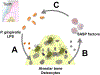
Cellular senescence is associated with inflammation and extracellular matrix tissue remodeling through the secretion of proteins termed the senescence-associated secretory phenotype (SASP). Although osteocyte senescence in older individuals in the skeleton is well recognized, whether young alveolar osteocytes can also become senescent is unknown. This is potentially important in the context of periodontal disease, which is an inflammatory condition caused by a gradual change from symbiotic to pathogenic oral microflora that can lead to tooth loss. Our aim was to identify whether senescent osteocytes accumulate in young alveolar bone and whether bacterial-derived lipopolysaccharide (LPS) can influence cellular senescence in alveolar bone. An osteocyte-enriched cell population isolated from alveolar bone expressed increased levels of the known senescence marker p16Ink4a, as well as select SASP markers known to be implicated alveolar bone resorption (Icam1, Il6, Il17, Mmp13 and Tnfα), compared to ramus control cells. Increased senescence of alveolar bone osteocytes was also observed in vivo using the senescence-associated distension of satellites (SADS) assay and increased γH2AX, a marker of DNA damage associated with senescent cells. To approximate a bacterial infection in vitro, alveolar osteocytes were treated with LPS. We found increased expression of various senescence and SASP markers, increased γH2AX staining, increased SA-β-Gal activity and the redistribution of F-actin leading to a larger and flattened cell morphology, all hallmarks of cellular senescence. In conclusion, our data suggests a model whereby bacterial-derived LPS stimulates premature alveolar osteocyte senescence, which in combination with the resultant SASP, could potentially contribute to the onset of alveolar bone loss.
Keywords: Alveolar bone; Bacteria; Inflammation; Osteocyte; Periodontal disease; SASP; Senescence.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

