Tau Positron Emission Tomographic Findings in a Former US Football Player With Pathologically Confirmed Chronic Traumatic Encephalopathy
- PMID: 31904765
- PMCID: PMC6990867
- DOI: 10.1001/jamaneurol.2019.4509
Tau Positron Emission Tomographic Findings in a Former US Football Player With Pathologically Confirmed Chronic Traumatic Encephalopathy
Abstract
Importance: Biomarkers for chronic traumatic encephalopathy (CTE) are currently lacking. The radiotracer fluorine F 18-labeled (18F)-flortaucipir (FTP) detects tau pathology in Alzheimer disease, and positron emission tomography (PET) with FTP shows elevated binding in individuals at risk for CTE. No study, however, has assessed the correlation between in vivo FTP PET and postmortem tau in CTE.
Objective: To assess the regional association between in vivo FTP binding and postmortem tau pathology in a patient with pathologically confirmed CTE.
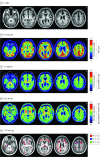
Design, setting, and participants: A white male former National Football League player with 17 years of US football exposure was clinically diagnosed with traumatic encephalopathy syndrome at a neurology tertiary referral center. 18F-Fludeoxyglucose, carbon 11-labeled Pittsburgh compound B, and FTP PET were performed 52 months prior to death, and magnetic resonance imaging, 50 months prior to death. Brain images were assessed qualitatively for abnormalities blinded to autopsy data. Autopsy was performed using a neurodegenerative research protocol. The FTP standardized uptake value ratios (inferior cerebellar gray reference region) and W-score (age-adjusted z-score) maps were compared with phosphorylated tau immunohistochemical analysis with monoclonal antibody CP13.
Main outcomes and measures: Qualitative and quantitative comparisons between antemortem FTP PET and tau pathology at autopsy.
Results: Flortaucipir uptake was distributed in a patchy, frontotemporal-predominant pattern that overlapped with regions showing neurodegeneration on magnetic resonance imaging and hypometabolism on 18F-fludeoxyglucose PET. Pathological assessment revealed stage 4 CTE; limbic argyrophilic grain disease; stage 2 limbic-predominant, age-related transactive response DNA-binding protein 43 encephalopathy; and Braak neurofibrillary tangle stage 3. 18F-Flortaucipir W-maps matched areas of high postmortem tau burden in left fusiform and inferior temporal gyri and juxtacortical frontal white matter. High FTP W-scores with low tau burden were found in the basal ganglia, thalamus, motor cortex, and calcarine cortex. No regions with low FTP W-scores corresponded to areas with high pathological tau burden. A modest correlation, which did not reach statistical significance (ρ = 0.35, P = .17), was found between FTP standardized uptake value ratio and tau area fraction at the regional level.
Conclusions and relevance: In this patient, FTP PET findings during life showed a modest correspondence with postmortem pathology in CTE. These findings suggest that FTP may have limited utility as a tau biomarker in CTE.
Conflict of interest statement
Figures

Comment in
-
Luftverschmutzung erhöht Demenzrisiko.MMW Fortschr Med. 2021 Feb;163(2):22. doi: 10.1007/s15006-021-9576-5. MMW Fortschr Med. 2021. PMID: 33527273 German. No abstract available.
References
-
- McKee AC, Cairns NJ, Dickson DW, et al. ; TBI/CTE group . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75-86. doi:10.1007/s00401-015-1515-z. doi:10.1007/s00401-015-1515-z - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

