MRP8/14 and neutrophil elastase for predicting treatment response and occurrence of flare in patients with juvenile idiopathic arthritis
- PMID: 31904851
- PMCID: PMC7449815
- DOI: 10.1093/rheumatology/kez590
MRP8/14 and neutrophil elastase for predicting treatment response and occurrence of flare in patients with juvenile idiopathic arthritis
Abstract
Objective: To study two neutrophil activation markers, myeloid-related protein (MRP) 8/14 and neutrophil elastase (NE), for their ability to predict treatment response and flare in patients with JIA.
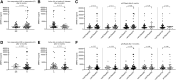
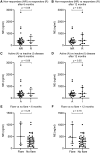
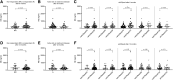
Methods: Using samples from two cohorts (I and II), we determined MRP8/14 and NE levels of 32 (I) and 81 (II) patients with new-onset, DMARD-naïve arthritis and compared patients who responded to treatment (defined as fulfilling ≥ adjusted ACRpedi50 response and/or inactive disease) with non-responders (defined as fulfilling < adjusted ACRpedi50 response and/or active disease) at 6 and 12 months. Secondly, we compared biomarker levels of 54 (I) and 34 (II) patients with clinically inactive disease who did or did not suffer from a flare of arthritis after 6 or 12 months. Receiver operating characteristic analyses were carried out to study the predictive value of MRP8/14 and NE for treatment response and flare.
Results: For both cohorts, baseline MRP8/14 and NE levels for patients who did or did not respond to treatment were not different. Also, MRP8/14 and NE levels were not different in patients who did or did not flare. Receiver operating characteristic analysis of MRP8/14 and NE demonstrated areas under the curve <0.7 in both cohorts.
Conclusion: In our cohorts, MRP8/14 and NE could not predict treatment response. Also, when patients had inactive disease, neither marker could predict flares.
Keywords: MRP8/14; S100A8/A9; biomarkers; calprotectin; disease activity; flare; juvenile idiopathic arthritis; neutrophil elastase; prediction; treatment response.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Thierry S, Fautrel B, Lemelle I, Guillemin F.. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine 2014;81:112–7. - PubMed
-
- Guzman J, Oen K, Tucker LB. et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854–60. - PubMed
-
- Bulatović M, Heijstek MW, Van Dijkhuizen EH. et al. Prediction of clinical non-response to methotrexate treatment in juvenile idiopathic arthritis. Ann Rheum Dis 2012;71:1484–9. - PubMed
-
- Aquilani A, Marafon DP, Marasco E. et al. Predictors of flare following etanercept withdrawal in patients with rheumatoid factor-negative juvenile idiopathic arthritis who reached remission while taking medication. J Rheumatol 2018;45:956–61. - PubMed
-
- Moncrieffe H, Ursu S, Holzinger D. et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 2013;52:1467–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

