CXCR4 or CXCR7 antagonists treat endometriosis by reducing bone marrow cell trafficking
- PMID: 31904910
- PMCID: PMC7028867
- DOI: 10.1111/jcmm.14933
CXCR4 or CXCR7 antagonists treat endometriosis by reducing bone marrow cell trafficking
Abstract
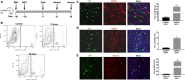
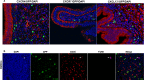
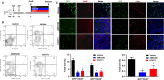
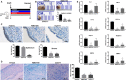
Adult stem cells have a major role in endometrial physiology, including remodelling and repair. However, they also have a critical role in the development and progression of endometriosis. Bone marrow-derived stem cells engraft eutopic endometrium and endometriotic lesions, differentiating to both stromal and epithelial cell fates. Using a mouse bone marrow transplantation model, we show that bone marrow-derived cells engrafting endometriosis express CXCR4 and CXCR7. Targeting either receptor by the administration of small molecule receptor antagonists AMD3100 or CCX771, respectively, reduced BM-derived stem cell recruitment into endometriosis implants. Endometriosis lesion size was decreased compared to vehicle controls after treatment with each antagonist in both an early growth and established lesion treatment model. Endometriosis lesion size was not effected when the local effects of CXCL12 were abrogated using uterine-specific CXCL12 null mice, suggesting an effect primarily on bone marrow cell migration rather than a direct endometrial effect. Antagonist treatment also decreased hallmarks of endometriosis physiopathology such as pro-inflammatory cytokine production and vascularization. CXCR4 and CXCR7 antagonists are potential novel, non-hormonal therapies for endometriosis.
Keywords: AMD3100; BMDSC; CCX771; CXCR4; CXCR7; bone marrow-derived stem cells; endometriosis.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures

Similar articles
-
Inhibition of CXCR4 and CXCR7 Is Protective in Acute Peritoneal Inflammation.Front Immunol. 2020 Mar 10;11:407. doi: 10.3389/fimmu.2020.00407. eCollection 2020. Front Immunol. 2020. PMID: 32210974 Free PMC article.
-
Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment.Endocrinology. 2014 Apr;155(4):1489-97. doi: 10.1210/en.2013-1977. Epub 2014 Jan 31. Endocrinology. 2014. PMID: 24484171 Free PMC article.
-
Endometriosis and Stem Cell Trafficking.Reprod Sci. 2016 Dec;23(12):1616-1619. doi: 10.1177/1933719116671219. Epub 2016 Oct 6. Reprod Sci. 2016. PMID: 27821558 Review.
-
Bone Marrow Stem Cell Chemotactic Activity Is Induced by Elevated CXCl12 in Endometriosis.Reprod Sci. 2017 Apr;24(4):526-533. doi: 10.1177/1933719116672587. Epub 2016 Oct 11. Reprod Sci. 2017. PMID: 27729562 Free PMC article.
-
Drug design strategies focusing on the CXCR4/CXCR7/CXCL12 pathway in leukemia and lymphoma.Expert Opin Drug Discov. 2016 Nov;11(11):1093-1109. doi: 10.1080/17460441.2016.1233176. Epub 2016 Sep 20. Expert Opin Drug Discov. 2016. PMID: 27598329 Review.
Cited by
-
Dual targeting of CXCR4 and EZH2 in endometriosis.iScience. 2025 Mar 1;28(4):112143. doi: 10.1016/j.isci.2025.112143. eCollection 2025 Apr 18. iScience. 2025. PMID: 40171488 Free PMC article.
-
Uterine Stem Cells and Benign Gynecological Disorders: Role in Pathobiology and Therapeutic Implications.Stem Cell Rev Rep. 2021 Jun;17(3):803-820. doi: 10.1007/s12015-020-10075-w. Epub 2020 Nov 5. Stem Cell Rev Rep. 2021. PMID: 33155150 Free PMC article. Review.
-
Loss of Cxcr4 in Endometriosis Reduces Proliferation and Lesion Number while Increasing Intraepithelial Lymphocyte Infiltration.Am J Pathol. 2021 Jul;191(7):1292-1302. doi: 10.1016/j.ajpath.2021.04.011. Epub 2021 May 6. Am J Pathol. 2021. PMID: 33964217 Free PMC article.
-
Endometriosis promotes atherosclerosis in a murine model.Am J Obstet Gynecol. 2022 Aug;227(2):248.e1-248.e8. doi: 10.1016/j.ajog.2022.03.040. Epub 2022 Mar 26. Am J Obstet Gynecol. 2022. PMID: 35351413 Free PMC article.
-
Advances in CXCR7 Modulators.Pharmaceuticals (Basel). 2020 Feb 21;13(2):33. doi: 10.3390/ph13020033. Pharmaceuticals (Basel). 2020. PMID: 32098047 Free PMC article. Review.
References
-
- Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update. 2013;19:625‐639. - PubMed
-
- Pluchino N, Wenger JM, Petignat P, et al. Sexual function in endometriosis patients and their partners: effect of the disease and consequences of treatment. Hum Reprod Update. 2016;22:762‐774. - PubMed
-
- Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395‐404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

