Reactions of Allylmagnesium Reagents with Carbonyl Compounds and Compounds with C═N Double Bonds: Their Diastereoselectivities Generally Cannot Be Analyzed Using the Felkin-Anh and Chelation-Control Models
- PMID: 31904936
- PMCID: PMC7018623
- DOI: 10.1021/acs.chemrev.9b00414
Reactions of Allylmagnesium Reagents with Carbonyl Compounds and Compounds with C═N Double Bonds: Their Diastereoselectivities Generally Cannot Be Analyzed Using the Felkin-Anh and Chelation-Control Models
Abstract
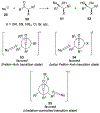
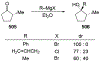
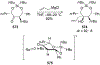
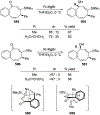
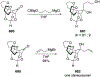
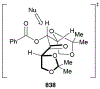
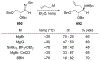
This review describes the additions of allylmagnesium reagents to carbonyl compounds and to imines, focusing on the differences in reactivity between allylmagnesium halides and other Grignard reagents. In many cases, allylmagnesium reagents either react with low stereoselectivity when other Grignard reagents react with high selectivity, or allylmagnesium reagents react with the opposite stereoselectivity. This review collects hundreds of examples, discusses the origins of stereoselectivities or the lack of stereoselectivity, and evaluates why selectivity may not occur and when it will likely occur.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




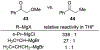
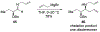
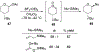
















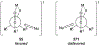



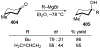
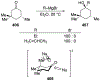
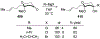
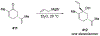




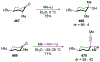
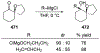

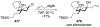



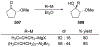


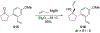
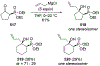

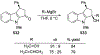
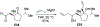
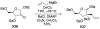
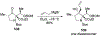

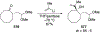
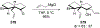





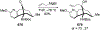
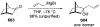
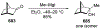



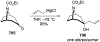
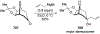
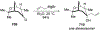
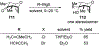






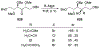



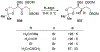


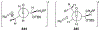
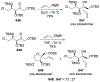
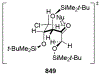
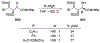
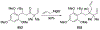


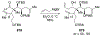
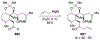
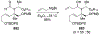
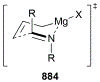
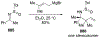
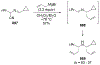

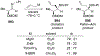





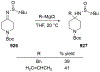

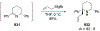
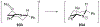
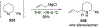

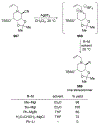
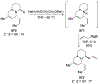
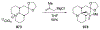
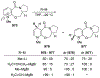











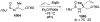
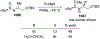

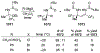

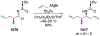







Similar articles
-
Allylmagnesium Halides Do Not React Chemoselectively Because Reaction Rates Approach the Diffusion Limit.J Org Chem. 2017 Feb 17;82(4):2300-2305. doi: 10.1021/acs.joc.7b00053. Epub 2017 Feb 8. J Org Chem. 2017. PMID: 28114754
-
Chelation-Controlled Additions to Chiral α- and β-Silyloxy, α-Halo, and β-Vinyl Carbonyl Compounds.Acc Chem Res. 2017 Sep 19;50(9):2389-2400. doi: 10.1021/acs.accounts.7b00319. Epub 2017 Aug 15. Acc Chem Res. 2017. PMID: 28809470
-
Asymmetric Direct 1,2-Addition of Aryl Grignard Reagents to Aryl Alkyl Ketones.Org Lett. 2016 Jan 15;18(2):236-9. doi: 10.1021/acs.orglett.5b03379. Epub 2015 Dec 24. Org Lett. 2016. PMID: 26704528
-
Enantioselective aldol reactions catalyzed by chiral phosphine oxides.Chem Rec. 2013 Aug;13(4):362-70. doi: 10.1002/tcr.201300004. Epub 2013 Jul 4. Chem Rec. 2013. PMID: 23828817 Review.
-
CuH-catalyzed reactions.Chem Rev. 2008 Aug;108(8):2916-27. doi: 10.1021/cr0684321. Epub 2008 Jul 11. Chem Rev. 2008. PMID: 18616323 Review. No abstract available.
Cited by
-
Controllable 1,3-Bis-Functionalization of 2-Nitroglycals with High Regioselectivity and Stereoselectivity Enabled by a H-Bond Catalyst.JACS Au. 2024 Mar 11;4(3):974-984. doi: 10.1021/jacsau.3c00727. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559736 Free PMC article.
-
The 100 facets of the Passerini reaction.Chem Sci. 2021 Sep 30;12(47):15445-15472. doi: 10.1039/d1sc03810a. eCollection 2021 Dec 8. Chem Sci. 2021. PMID: 35003575 Free PMC article. Review.
-
Evidence against Single-Electron Transfer in the Additions of Most Organomagnesium Reagents to Carbonyl Compounds.J Org Chem. 2020 Jun 19;85(12):7848-7862. doi: 10.1021/acs.joc.0c00481. Epub 2020 May 28. J Org Chem. 2020. PMID: 32407636 Free PMC article.
-
Biomimetic Synthesis of Azorellolide via Cyclopropylcarbinyl Cation Chemistry.J Am Chem Soc. 2025 Jan 8;147(1):78-83. doi: 10.1021/jacs.4c14664. Epub 2024 Dec 18. J Am Chem Soc. 2025. PMID: 39693250 Free PMC article.
-
Unmasking the halide effect in diastereoselective Grignard reactions applied to C4´ modified nucleoside synthesis.Nat Commun. 2025 Feb 16;16(1):1679. doi: 10.1038/s41467-025-56895-7. Nat Commun. 2025. PMID: 39956836 Free PMC article.
References
-
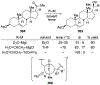
- Yamamoto Y; Asao N Selective Reactions Using Allylic Metals. Chem. Rev 1993, 93, 2207–2293.
-
- Tatsuta K; Fukuda T; Ishimori T; Yachi R; Yoshida S; Hashimoto H; Hosokawa S The First Total Synthesis of Hibarimicinone, a Potent v-Src Tyrosine Kinase Inhibitor. Tetrahedron Lett. 2012, 53, 422–425.
-
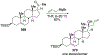
- Bejjani J; Chemla F; Audouin M N-Tritylprolinal: An Efficient Building Block for the Stereoselective Synthesis of Proline-Derived Amino Alcohols. J. Org. Chem 2003, 68, 9747–9752. - PubMed
-
- Carda M; González F; Rodríguez S; Marco JA Highly Diastereoselective Additions of Organometallic Reagents to 1-O-Silylated 3,4-Di-O-Benzyl-l-Erythrulose Derivatives. Tetrahedron: Asymmetry 1993, 4, 1799–1802.
-
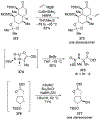
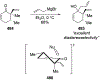
- Holt DJ; Barker WD; Jenkins PR; Davies DL; Garratt S; Fawcett J; Russell DR; Ghosh S Ring-Closing Metathesis in Carbohydrate Annulation. Angew. Chem. Int. Ed 1998, 37, 3298–3300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

