Tricyclic Imidazolidin-4-ones by Witkop Oxidation of Tetrahydro-β-carbolines
- PMID: 31904963
- PMCID: PMC7092372
- DOI: 10.1021/acs.joc.9b03402
Tricyclic Imidazolidin-4-ones by Witkop Oxidation of Tetrahydro-β-carbolines
Abstract
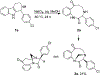
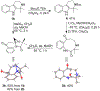
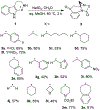
1-Substituted and 1,1-disubstituted tetrahydro-β-carbolines undergo sodium periodate oxidative ring expansion in the presence of formaldehyde and other aldehydes to form 5,6-dihydro-7H-1,4-methanobenzo[e][1,4]diazonine-2,7(3H)-diones in 30-81% yield. In most cases, the reaction to form this new 6/8/5-tricyclic ring system proceeds with high diastereoselectivity. These benzannulated medium-ring keto imidazolidin-4-ones expand the menu of tetrahydro-β-carboline oxidation products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

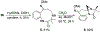
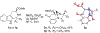

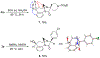
References
-
- Roxburgh CJ Syntheses of medium sized rings by ring expansion reactions. Tetrahedron 1993, 49, 10749–10784.
-
- Donald JR; Unsworth WP Ring-expansion reactions in the synthesis of macrocycles and medium-sized rings. Chem. - Eur. J 2017, 23, 8780–8799. - PubMed
-
- Stach H; Hesse M Synthesis of macrocyclic compounds by ring enlargement. Tetrahedron 1988, 44, 1573–1590.
-
- Witkop B; James B; Patrick JB; Rosenblum MJ Ring effects in autoxidation. A new type of Camps reaction. J. Am. Chem. Soc 1951, 73 (6), 2641–2647.
- Mentel M; Breinbauer R The Witkop-Winterfeldt oxidation of indoles. Curr. Org. Chem 2007, 11, 159–176.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

