Crimean-Congo hemorrhagic fever virus replication imposes hyper-lipidation of MAP1LC3 in epithelial cells
- PMID: 31905032
- PMCID: PMC8386629
- DOI: 10.1080/15548627.2019.1709765
Crimean-Congo hemorrhagic fever virus replication imposes hyper-lipidation of MAP1LC3 in epithelial cells
Abstract
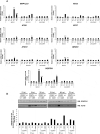
Crimean-Congo hemorrhagic fever virus (CCHFV) is a virus that causes severe liver dysfunctions and hemorrhagic fever, with high mortality rate. Here, we show that CCHFV infection caused a massive lipidation of LC3 in hepatocytes. This lipidation was not dependent on ATG5, ATG7 or BECN1, and no signs for recruitment of the alternative ATG12-ATG3 pathway for lipidation was found. Both virus replication and protein synthesis were required for the lipidation of LC3. Despite an augmented transcription of SQSTM1, the amount of proteins did not show a massive and sustained increase in infected cells, indicating that degradation of SQSTM1 by macroautophagy/autophagy was still occurring. The genetic alteration of autophagy did not influence the production of CCHFV particles demonstrating that autophagy was not required for CCHFV replication. Thus, the results indicate that CCHFV multiplication imposes an overtly elevated level of LC3 mobilization that involves a possibly novel type of non-canonical lipidation. Abbreviations: BECN1: Beclin 1; CCHF: Crimean-Congo hemorrhagic fever; CCHFV: Crimean-Congo hemorrhagic fever virus; CHX: cycloheximide; ER: endoplasmic reticulum; GFP: green fluorescent protein; GP: glycoproteins; MAP1LC3: microtubule associated protein 1 light chain 3; MOI: multiplicity of infection; n.i.: non-infected; NP: nucleoprotein; p.i.: post-infection; SQSTM1: sequestosome 1.
Keywords: Autophagy; CCHFV; LC3 lipidation; epithelial cells; viral infection.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
-
- Whitehouse CA.Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64(3):145–160. - PubMed
-
- Bente DA, Forrester NL, Watts DM, et al. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100(1):159–189. - PubMed
-
- Vorou R, Pierroutsakos IN, Maltezou HC. Crimean-Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20(5):495–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
