Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Phase 3, Randomized, Noninferiority TANGO Study
- PMID: 31905383
- PMCID: PMC7643745
- DOI: 10.1093/cid/ciz1243
Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Phase 3, Randomized, Noninferiority TANGO Study
Abstract
Background: The 2-drug regimen dolutegravir (DTG) + lamivudine (3TC) is indicated for treatment-naive adults with human immunodeficiency virus type 1 (HIV-1). We present efficacy and safety of switching to DTG/3TC in virologically suppressed individuals.
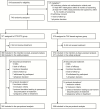
Methods: TANGO is an open-label, multicenter, phase 3 study that randomized adults (1:1, stratified by baseline third agent class) with HIV-1 RNA <50 copies/mL to switch to once-daily fixed-dose DTG/3TC or remain on a tenofovir alafenamide (TAF)-based regimen. The primary end point was proportion of participants with HIV-1 RNA ≥50 copies/mL at week 48 (US Food and Drug Administration Snapshot algorithm) in the intention-to-treat-exposed population (4% noninferiority margin).
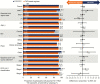
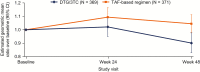
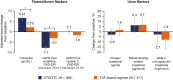
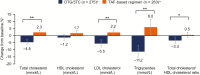
Results: 743 adults were enrolled; 741 received ≥1 dose of study drug (DTG/3TC, N = 369; TAF-based regimen, N = 372). At week 48, proportion of participants with HIV-1 RNA ≥50 copies/mL receiving DTG/3TC was 0.3% (1/369) vs 0.5% (2/372) with a TAF-based regimen (adjusted treatment difference [95% confidence interval], -0.3 [-1.2 to .7]), meeting noninferiority criteria. No participants receiving DTG/3TC and 1 receiving a TAF-based regimen met confirmed virologic withdrawal criteria, with no emergent resistance at failure. Drug-related grade ≥2 adverse events and withdrawals due to adverse events occurred in 17 (4.6%) and 13 (3.5%) participants with DTG/3TC and 3 (0.8%) and 2 (0.5%) with a TAF-based regimen, respectively.
Conclusions: DTG/3TC was noninferior in maintaining virologic suppression vs a TAF-based regimen at week 48, with no virologic failure or emergent resistance reported with DTG/3TC, supporting it as a simplification strategy for virologically suppressed people with HIV-1.
Clinical trials registration: NCT03446573.
Keywords: 2-drug regimen; integrase strand transfer inhibitor; nucleoside reverse transcriptase inhibitor; simplification; virologic suppression.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Kelly SG, Nyaku AN, Taiwo BO. Two-drug treatment approaches in HIV: finally getting somewhere? Drugs 2016; 76:523–31. - PubMed
-
- Juluca [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2019.
-
- Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

