Effects of Influenza Vaccination in the United States During the 2018-2019 Influenza Season
- PMID: 31905401
- PMCID: PMC7643742
- DOI: 10.1093/cid/ciz1244
Effects of Influenza Vaccination in the United States During the 2018-2019 Influenza Season
Abstract
Background: Multivalent influenza vaccine products provide protection against influenza A(H1N1)pdm09, A(H3N2), and B lineage viruses. The 2018-2019 influenza season in the United States included prolonged circulation of A(H1N1)pdm09 viruses well-matched to the vaccine strain and A(H3N2) viruses, the majority of which were mismatched to the vaccine. We estimated the number of vaccine-prevented influenza-associated illnesses, medical visits, hospitalizations, and deaths for the season.
Methods: We used a mathematical model and Monte Carlo algorithm to estimate numbers and 95% uncertainty intervals (UIs) of influenza-associated outcomes prevented by vaccination in the United States. The model incorporated age-specific estimates of national 2018-2019 influenza vaccine coverage, influenza virus-specific vaccine effectiveness from the US Influenza Vaccine Effectiveness Network, and disease burden estimated from population-based rates of influenza-associated hospitalizations through the Influenza Hospitalization Surveillance Network.
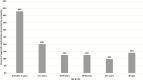
Results: Influenza vaccination prevented an estimated 4.4 million (95%UI, 3.4 million-7.1 million) illnesses, 2.3 million (95%UI, 1.8 million-3.8 million) medical visits, 58 000 (95%UI, 30 000-156 000) hospitalizations, and 3500 (95%UI, 1000-13 000) deaths due to influenza viruses during the US 2018-2019 influenza season. Vaccination prevented 14% of projected hospitalizations associated with A(H1N1)pdm09 overall and 43% among children aged 6 months-4 years.
Conclusions: Influenza vaccination averted substantial influenza-associated disease including hospitalizations and deaths in the United States, primarily due to effectiveness against A(H1N1)pdm09. Our findings underscore the value of influenza vaccination, highlighting that vaccines measurably decrease illness and associated healthcare utilization even in a season in which a vaccine component does not match to a circulating virus.
Keywords: burden; influenza; prevented illnesses; vaccination.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Figures
References
-
- Centers for Disease Control and Prevention. 2018–2019 US flu season: preliminary burden estimates Available at: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm. Accessed 15 October 2019.
-
- Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2018–19 influenza season Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed 21 October 2019.
-
- Belongia EA, Simpson MD, King JP, et al. . Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

