Skeletal mineralization: mechanisms and diseases
- PMID: 31905439
- PMCID: PMC6944863
- DOI: 10.6065/apem.2019.24.4.213
Skeletal mineralization: mechanisms and diseases
Abstract
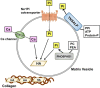
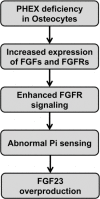
Skeletal mineralization is initiated in matrix vesicles (MVs), the small extracellular vesicles derived from osteoblasts and chondrocytes. Calcium and inorganic phosphate (Pi) taken up by MVs form hydroxyapatite crystals, which propagate on collagen fibrils to mineralize the extracellular matrix. Insufficient calcium or phosphate impairs skeletal mineralization. Because active vitamin D is necessary for intestinal calcium absorption, vitamin D deficiency is a significant cause of rickets/osteomalacia. Chronic hypophosphatemia also results in rickets/osteomalacia. Excessive action of fibroblast growth factor 23 (FGF23), a key regulator of Pi metabolism, leads to renal Pi wasting and impairs vitamin D activation. X-linked hypophosphatemic rickets (XLH) is the most common form of hereditary FGF23-related hypophosphatemia, and enhanced FGF receptor (FGFR) signaling in osteocytes may be involved in the pathogenesis of this disease. Increased extracellular Pi triggers signal transduction via FGFR to regulate gene expression, implying a close relationship between Pi metabolism and FGFR. An anti-FGF23 antibody, burosumab, has recently been developed as a new treatment for XLH. In addition to various forms of rickets/osteomalacia, hypophosphatasia (HPP) is characterized by impaired skeletal mineralization. HPP is caused by inactivating mutations in tissue-nonspecific alkaline phosphatase, an enzyme rich in MVs. The recent development of enzyme replacement therapy using bone-targeting recombinant alkaline phosphatase has improved the prognosis, motor function, and quality of life in patients with HPP. This links impaired skeletal mineralization with various conditions, and unraveling its pathogenesis will lead to more precise diagnoses and effective treatments.
Keywords: Hypophosphatasia; Phosphate; Rickets; Vitamin D; Skeletal mineralization.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Fukumoto S, Ozono K, Michigami T, Minagawa M, Okazaki R, Sugimoto T, et al. Pathogenesis and diagnostic criteria for rickets and osteomalacia--proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J Bone Miner Metab. 2015;33:467–73. - PubMed
-
- Millán JL. What can we learn about the neural functions of TNAP from studies on other organs and tissues? Subcell Biochem. 2015;76:155–66. - PubMed
-
- Whyte MP. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12:233–46. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

