Difference in Developmental Kinetics of Y-Specific Monoclonal Antibody Sorted Male and Female In Vitro Produced Bovine Embryos
- PMID: 31905822
- PMCID: PMC6981608
- DOI: 10.3390/ijms21010244
Difference in Developmental Kinetics of Y-Specific Monoclonal Antibody Sorted Male and Female In Vitro Produced Bovine Embryos
Abstract
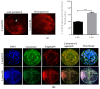
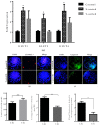
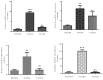
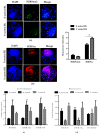
Sex-related growth differences between male and female embryos remain an attractive subject for reproductive biologists. This study aimed to investigate the endogenous factors that play a crucial role in the pace of early development between male and female bovine embryos. Using sex pre-selected semen by Y-specific monoclonal antibodies for the production of bovine embryos, we characterized the critical endogenous factors that are responsible for creating the development differences, especially during the pre-implantation period between male and female embryos. Our results showed that at day seven, (57.8%) Y-sperm sorted in vitro cultured embryos reached the expanded blastocyst (BL) stage, whereas the X-sperm sorted group were only 25%. Y-BLs showed higher mRNA abundance of pluripotency and developmental competency regulators, such as Oct4 and IGF1-R. Interestingly, Y-sperm sorted BLs had a homogeneous mitochondrial distribution pattern, higher mitochondrial membrane potential (∆Ѱm), efficient OXPHOS (oxidative phosphorylation) system and well-encountered production of ROS (reactive oxygen species) level. Moreover, Y-blastocysts (BLs) showed less utilization of glucose metabolism relative to the X-BLs group. Importantly, both sexes showed differences in the timing of epigenetic events. All these factors directly or indirectly orchestrate the whole embryonic progression and may help in the faster and better quality yield of BL in the Y-sperm sorted group compared to the X counterpart group.
Keywords: Bovine blastocyst; Y-specific monoclonal antibody; developmental kinetics; sex differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

