Vertically-Aligned Functionalized Silicon Micropillars for 3D Culture of Human Pluripotent Stem Cell-Derived Cortical Progenitors
- PMID: 31905823
- PMCID: PMC7017050
- DOI: 10.3390/cells9010088
Vertically-Aligned Functionalized Silicon Micropillars for 3D Culture of Human Pluripotent Stem Cell-Derived Cortical Progenitors
Abstract
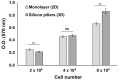
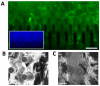
Silicon is a promising material for tissue engineering since it allows to produce micropatterned scaffolding structures resembling biological tissues. Using specific fabrication methods, it is possible to build aligned 3D network-like structures. In the present study, we exploited vertically-aligned silicon micropillar arrays as culture systems for human iPSC-derived cortical progenitors. In particular, our aim was to mimic the radially-oriented cortical radial glia fibres that during embryonic development play key roles in controlling the expansion, radial migration and differentiation of cortical progenitors, which are, in turn, pivotal to the establishment of the correct multilayered cerebral cortex structure. Here we show that silicon vertical micropillar arrays efficiently promote expansion and stemness preservation of human cortical progenitors when compared to standard monolayer growth conditions. Furthermore, the vertically-oriented micropillars allow the radial migration distinctive of cortical progenitors in vivo. These results indicate that vertical silicon micropillar arrays can offer an optimal system for human cortical progenitors' growth and migration. Furthermore, similar structures present an attractive platform for cortical tissue engineering.
Keywords: 3D culture; cell growth; cerebral cortex; hiPSC-derived neural progenitors; human cortical progenitors; silicon pillars.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

