Neuroprotective Effects of Quercetin in Alzheimer's Disease
- PMID: 31905923
- PMCID: PMC7023116
- DOI: 10.3390/biom10010059
Neuroprotective Effects of Quercetin in Alzheimer's Disease
Abstract
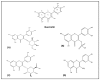
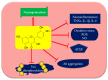
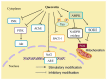
Quercetin is a flavonoid with notable pharmacological effects and promising therapeutic potential. It is widely distributed among plants and found commonly in daily diets predominantly in fruits and vegetables. Neuroprotection by quercetin has been reported in several in vitro studies. It has been shown to protect neurons from oxidative damage while reducing lipid peroxidation. In addition to its antioxidant properties, it inhibits the fibril formation of amyloid-β proteins, counteracting cell lyses and inflammatory cascade pathways. In this review, we provide a synopsis of the recent literature exploring the relationship between quercetin and cognitive performance in Alzheimer's disease and its potential as a lead compound in clinical applications.
Keywords: Alzheimer’s disease; clinical directions; mechanistic insights; polyphenols; quercetin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Donepezil-butylated hydroxytoluene (BHT) hybrids as Anti-Alzheimer's disease agents with cholinergic, antioxidant, and neuroprotective properties.Eur J Med Chem. 2018 Sep 5;157:161-176. doi: 10.1016/j.ejmech.2018.08.005. Epub 2018 Aug 4. Eur J Med Chem. 2018. PMID: 30096650
-
Recent Developments on Multi-Target-Directed Tacrines for Alzheimer's Disease. I. The Pyranotacrines.Curr Top Med Chem. 2017;17(31):3328-3335. doi: 10.2174/1568026618666180112155639. Curr Top Med Chem. 2017. PMID: 29332586 Review.
-
Development of cyanopyridine-triazine hybrids as lead multitarget anti-Alzheimer agents.Bioorg Med Chem. 2016 Jun 15;24(12):2777-88. doi: 10.1016/j.bmc.2016.04.041. Epub 2016 Apr 22. Bioorg Med Chem. 2016. PMID: 27157006
-
Novel salicylamide derivatives as potent multifunctional agents for the treatment of Alzheimer's disease: Design, synthesis and biological evaluation.Bioorg Chem. 2019 Mar;84:137-149. doi: 10.1016/j.bioorg.2018.11.022. Epub 2018 Nov 22. Bioorg Chem. 2019. PMID: 30500523
-
Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease.Acc Chem Res. 2021 Oct 19;54(20):3930-3940. doi: 10.1021/acs.accounts.1c00457. Epub 2021 Oct 4. Acc Chem Res. 2021. PMID: 34606227 Review.
Cited by
-
The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer's Disease: Therapeutic Implications and Future Perspectives.Antioxidants (Basel). 2022 Oct 31;11(11):2167. doi: 10.3390/antiox11112167. Antioxidants (Basel). 2022. PMID: 36358538 Free PMC article. Review.
-
Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer's disease.J Neuroinflammation. 2022 Aug 17;19(1):206. doi: 10.1186/s12974-022-02565-0. J Neuroinflammation. 2022. PMID: 35978311 Free PMC article. Review.
-
Targeting Mitochondria by Plant Secondary Metabolites: A Promising Strategy in Combating Parkinson's Disease.Int J Mol Sci. 2021 Nov 22;22(22):12570. doi: 10.3390/ijms222212570. Int J Mol Sci. 2021. PMID: 34830453 Free PMC article. Review.
-
B serum proteome profiles revealed dysregulated proteins and mechanisms associated with insomnia patients: A preliminary study.Front Integr Neurosci. 2022 Jul 26;16:936955. doi: 10.3389/fnint.2022.936955. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35958162 Free PMC article.
-
Pharmacokinetic studies, molecular docking, and molecular dynamics simulations of phytochemicals from Morus alba: a multi receptor approach for potential therapeutic agents in colorectal cancer.Med Oncol. 2024 May 15;41(6):156. doi: 10.1007/s12032-024-02406-5. Med Oncol. 2024. PMID: 38750377
References
-
- Kommaddi R.P., Das D., Karunakaran S., Nanguneri S., Bapat D., Ray A., Shaw E., Bennett D.A., Nair D., Ravindranath V. Aβ mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer’s disease. J. Neurosci. 2018;38:1085–1099. doi: 10.1523/JNEUROSCI.2127-17.2017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

