Steroid Therapy and Steroid Response in Autoimmune Pancreatitis
- PMID: 31905944
- PMCID: PMC6981453
- DOI: 10.3390/ijms21010257
Steroid Therapy and Steroid Response in Autoimmune Pancreatitis
Abstract
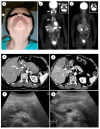
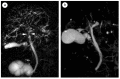
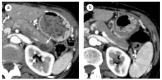
Autoimmune pancreatitis (AIP), a unique subtype of pancreatitis, is often accompanied by systemic inflammatory disorders. AIP is classified into two distinct subtypes on the basis of the histological subtype: immunoglobulin G4 (IgG4)-related lymphoplasmacytic sclerosing pancreatitis (type 1) and idiopathic duct-centric pancreatitis (type 2). Type 1 AIP is often accompanied by systemic lesions, biliary strictures, hepatic inflammatory pseudotumors, interstitial pneumonia and nephritis, dacryoadenitis, and sialadenitis. Type 2 AIP is associated with inflammatory bowel diseases in approximately 30% of cases. Standard therapy for AIP is oral corticosteroid administration. Steroid treatment is generally indicated for symptomatic cases and is exceptionally applied for cases with diagnostic difficulty (diagnostic steroid trial) after a negative workup for malignancy. More than 90% of patients respond to steroid treatment within 1 month, and most within 2 weeks. The steroid response can be confirmed on clinical images (computed tomography, ultrasonography, endoscopic ultrasonography, magnetic resonance imaging, and 18F-fluorodeoxyglucose-positron emission tomography). Hence, the steroid response is included as an optional diagnostic item of AIP. Steroid treatment results in normalization of serological markers, including IgG4. Short- and long-term corticosteroid treatment may induce adverse events, including chronic glycometabolism, obesity, an immunocompromised status against infection, cataracts, glaucoma, osteoporosis, and myopathy. AIP is common in old age and is often associated with diabetes mellitus (33-78%). Thus, there is an argument for corticosteroid therapy in diabetes patients with no symptoms. With low-dose steroid treatment or treatment withdrawal, there is a high incidence of AIP recurrence (24-52%). Therefore, there is a need for long-term steroid maintenance therapy and/or steroid-sparing agents (immunomodulators and rituximab). Corticosteroids play a critical role in the diagnosis and treatment of AIP.
Keywords: IgG4; autoimmune pancreatitis; corticosteroid; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
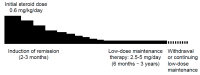

Similar articles
-
Autoimmune Pancreatitis: From Pathogenesis to Treatment.Int J Mol Sci. 2022 Oct 21;23(20):12667. doi: 10.3390/ijms232012667. Int J Mol Sci. 2022. PMID: 36293522 Free PMC article. Review.
-
Autoimmune pancreatitis: an update.Abdom Radiol (NY). 2020 May;45(5):1359-1370. doi: 10.1007/s00261-019-02275-x. Abdom Radiol (NY). 2020. PMID: 31650376 Review.
-
Autoimmune Pancreatitis: An Update on Diagnosis and Management.Gastroenterol Clin North Am. 2016 Mar;45(1):29-43. doi: 10.1016/j.gtc.2015.10.005. Gastroenterol Clin North Am. 2016. PMID: 26895679 Review.
-
Differentiation of focal autoimmune pancreatitis from pancreatic ductal adenocarcinoma.Abdom Radiol (NY). 2020 May;45(5):1371-1386. doi: 10.1007/s00261-019-02210-0. Abdom Radiol (NY). 2020. PMID: 31493022 Review.
-
Impact of sclerosing dacryoadenitis/sialadenitis on relapse during steroid therapy in patients with type 1 autoimmune pancreatitis.Scand J Gastroenterol. 2019 Feb;54(2):259-264. doi: 10.1080/00365521.2019.1577489. Epub 2019 Mar 27. Scand J Gastroenterol. 2019. PMID: 30915865
Cited by
-
Diagnosis and Management of Acute Pancreatitis.Diagnostics (Basel). 2025 Jan 23;15(3):258. doi: 10.3390/diagnostics15030258. Diagnostics (Basel). 2025. PMID: 39941188 Free PMC article. Review.
-
IgG4-Related Sclerosing Cholangitis: Rarely Diagnosed, but not a Rare Disease.Can J Gastroenterol Hepatol. 2021 Dec 21;2021:1959832. doi: 10.1155/2021/1959832. eCollection 2021. Can J Gastroenterol Hepatol. 2021. PMID: 34970512 Free PMC article. Review.
-
Turkish Society of Gastroenterology: Pancreas Working Group, Acute Pancreatitis Committee Consensus Report.Turk J Gastroenterol. 2024 Nov 11;35(Suppl 1):S1-S44. doi: 10.5152/tjg.2024.24392. Turk J Gastroenterol. 2024. PMID: 39599919 Free PMC article. Review.
-
A tale of two pancreases: exocrine pathology and endocrine dysfunction.Diabetologia. 2020 Oct;63(10):2030-2039. doi: 10.1007/s00125-020-05210-8. Epub 2020 Sep 7. Diabetologia. 2020. PMID: 32894313 Free PMC article. Review.
-
Unraveling the relationship between autoimmune pancreatitis type 2 and inflammatory bowel disease: Results from two centers and systematic review of the literature.United European Gastroenterol J. 2022 Jun;10(5):496-506. doi: 10.1002/ueg2.12237. Epub 2022 May 8. United European Gastroenterol J. 2022. PMID: 35526270 Free PMC article.
References
-
- Shimosegawa T., Chari S.T., Frulloni L., Kamisawa T., Kawa S., Mino-Kenudson M., Kim M.-H., Kippel G., Lerch M.M., Lhr M., et al. International Consensus Diagnostic Criteria for autoimmune pancreatitis—Guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. - DOI - PubMed
-
- Kawa S., Okazaki K., Kamisawa T., Shimosegawa T., Tanaka M., Working members of Research Committee for Intractable Pancreatic Disease and Japan Pancreas Society Japanese consensus guidelines for management of autoimmune pancreatitis: II. Extrapancreatic lesions, differential diagnosis. J. Gastroenterol. 2010;45:355–369. doi: 10.1007/s00535-009-0197-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

