Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia
- PMID: 31905962
- PMCID: PMC7022278
- DOI: 10.3390/biom10010061
Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia
Abstract
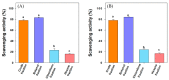
Nuxia oppositifolia is traditionally used in diabetes treatment in many Arabian countries; however, scientific evidence is lacking. Hence, the present study explored the antidiabetic and antioxidant activities of the plant extracts and their purified compounds. The methanolic crude extract of N. oppositifolia was partitioned using a two-solvent system. The n-hexane fraction was purified by silica gel column chromatography to yield several compounds including katononic acid and 3-oxolupenal. Antidiabetic activities were assessed by α-amylase and α-glucosidase enzyme inhibition. Antioxidant capacities were examined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) scavenging assays. Further, the interaction between enzymes (α-amylase and α-glucosidase) and ligands (3-oxolupenal and katononic acid) was followed by fluorescence quenching and molecular docking studies. 3-oxolupenal and katononic acid showed IC50 values of 46.2 μg/mL (101.6 µM) and 52.4 μg/mL (119.3 µM), respectively against the amylase inhibition. 3-oxolupenal (62.3 µg/mL or 141.9 μM) exhibited more potent inhibition against α-glucosidases compared to katononic acid (88.6 µg/mL or 194.8 μM). In terms of antioxidant activity, the relatively polar crude extract and n-butanol fraction showed the greatest DPPH and ABTS scavenging activity. However, the antioxidant activities of the purified compounds were in the low to moderate range. Molecular docking studies confirmed that 3-oxolupenal and katononic acid interacted strongly with the active site residues of both α-amylase and α-glucosidase. Fluorescence quenching results also suggest that 3-oxolupenal and katononic acid have a good affinity towards both α-amylase and α-glucosidase enzymes. This study provides preliminary data for the plant's use in the treatment of type 2 diabetes mellitus.
Keywords: 3-oxolupenal; ABTS; DPPH; Nuxia oppositifolia; amylase; antioxidant; docking; glucosidase; katononic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

