Cell-to-Cell Communication in Learning and Memory: From Neuro- and Glio-Transmission to Information Exchange Mediated by Extracellular Vesicles
- PMID: 31906013
- PMCID: PMC6982255
- DOI: 10.3390/ijms21010266
Cell-to-Cell Communication in Learning and Memory: From Neuro- and Glio-Transmission to Information Exchange Mediated by Extracellular Vesicles
Abstract
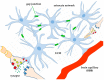
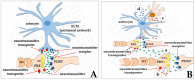
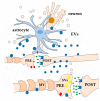
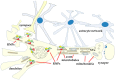
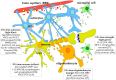
Most aspects of nervous system development and function rely on the continuous crosstalk between neurons and the variegated universe of non-neuronal cells surrounding them. The most extraordinary property of this cellular community is its ability to undergo adaptive modifications in response to environmental cues originating from inside or outside the body. Such ability, known as neuronal plasticity, allows long-lasting modifications of the strength, composition and efficacy of the connections between neurons, which constitutes the biochemical base for learning and memory. Nerve cells communicate with each other through both wiring (synaptic) and volume transmission of signals. It is by now clear that glial cells, and in particular astrocytes, also play critical roles in both modes by releasing different kinds of molecules (e.g., D-serine secreted by astrocytes). On the other hand, neurons produce factors that can regulate the activity of glial cells, including their ability to release regulatory molecules. In the last fifteen years it has been demonstrated that both neurons and glial cells release extracellular vesicles (EVs) of different kinds, both in physiologic and pathological conditions. Here we discuss the possible involvement of EVs in the events underlying learning and memory, in both physiologic and pathological conditions.
Keywords: extracellular vesicles; glial cells; learning; memory; synaptic plasticity; tetrapartite synapse; tripartite synapsis; volume transmission; wiring transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[The updated advancements in synaptic plasticity mediated by glial cells].Sheng Li Ke Xue Jin Zhan. 2007 Apr;38(2):111-5. Sheng Li Ke Xue Jin Zhan. 2007. PMID: 17633222 Review. Chinese.
-
Glia: the many ways to modulate synaptic plasticity.Neurochem Int. 2010 Nov;57(4):440-5. doi: 10.1016/j.neuint.2010.02.013. Epub 2010 Mar 1. Neurochem Int. 2010. PMID: 20193723 Review.
-
Glial cells in synaptic plasticity.J Physiol Paris. 2006 Mar-May;99(2-3):75-83. doi: 10.1016/j.jphysparis.2005.12.002. Epub 2006 Jan 30. J Physiol Paris. 2006. PMID: 16446078 Review.
-
Plasticity of Neuron-Glial Transmission: Equipping Glia for Long-Term Integration of Network Activity.Neural Plast. 2015;2015:765792. doi: 10.1155/2015/765792. Epub 2015 Aug 3. Neural Plast. 2015. PMID: 26339509 Free PMC article. Review.
-
Glia-neuron intercommunications and synaptic plasticity.Prog Neurobiol. 1996 Jun;49(3):185-214. doi: 10.1016/s0301-0082(96)00012-3. Prog Neurobiol. 1996. PMID: 8878303 Review.
Cited by
-
Isolation and Enrichment of Major Primary Neuroglial Cells from Neonatal Mouse Brain.Bio Protoc. 2024 Jan 20;14(2):e4921. doi: 10.21769/BioProtoc.4921. eCollection 2024 Jan 20. Bio Protoc. 2024. PMID: 38268978 Free PMC article.
-
Extracellular vesicles (exosomes and ectosomes) play key roles in the pathology of brain diseases.Mol Biomed. 2021 Jun 20;2(1):18. doi: 10.1186/s43556-021-00040-5. Mol Biomed. 2021. PMID: 35006460 Free PMC article. Review.
-
Reduction of eEF2 kinase alleviates the learning and memory impairment caused by acrylamide.Cell Biosci. 2024 Aug 23;14(1):106. doi: 10.1186/s13578-024-01285-7. Cell Biosci. 2024. PMID: 39180059 Free PMC article.
-
RNA-Binding Proteins as Epigenetic Regulators of Brain Functions and Their Involvement in Neurodegeneration.Int J Mol Sci. 2022 Nov 23;23(23):14622. doi: 10.3390/ijms232314622. Int J Mol Sci. 2022. PMID: 36498959 Free PMC article. Review.
-
Protein acetylation regulates xylose metabolism during adaptation of Saccharomyces cerevisiae.Biotechnol Biofuels. 2021 Dec 17;14(1):241. doi: 10.1186/s13068-021-02090-x. Biotechnol Biofuels. 2021. PMID: 34920742 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

