On the Modulatory Roles of Neuregulins/ErbB Signaling on Synaptic Plasticity
- PMID: 31906113
- PMCID: PMC6981567
- DOI: 10.3390/ijms21010275
On the Modulatory Roles of Neuregulins/ErbB Signaling on Synaptic Plasticity
Abstract
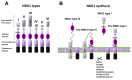
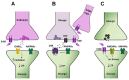
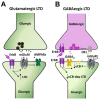
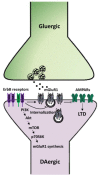
Neuregulins (NRGs) are a family of epidermal growth factor-related proteins, acting on tyrosine kinase receptors of the ErbB family. NRGs play an essential role in the development of the nervous system, since they orchestrate vital functions such as cell differentiation, axonal growth, myelination, and synapse formation. They are also crucially involved in the functioning of adult brain, by directly modulating neuronal excitability, neurotransmission, and synaptic plasticity. Here, we provide a review of the literature documenting the roles of NRGs/ErbB signaling in the modulation of synaptic plasticity, focusing on evidence reported in the hippocampus and midbrain dopamine (DA) nuclei. The emerging picture shows multifaceted roles of NRGs/ErbB receptors, which critically modulate different forms of synaptic plasticity (LTP, LTD, and depotentiation) affecting glutamatergic, GABAergic, and DAergic synapses, by various mechanisms. Further, we discuss the relevance of NRGs/ErbB-dependent synaptic plasticity in the control of brain processes, like learning and memory and the known involvement of NRGs/ErbB signaling in the modulation of synaptic plasticity in brain's pathological conditions. Current evidence points to a central role of NRGs/ErbB receptors in controlling glutamatergic LTP/LTD and GABAergic LTD at hippocampal CA3-CA1 synapses, as well as glutamatergic LTD in midbrain DA neurons, thus supporting that NRGs/ErbB signaling is essential for proper brain functions, cognitive processes, and complex behaviors. This suggests that dysregulated NRGs/ErbB-dependent synaptic plasticity might contribute to mechanisms underlying different neurological and psychiatric disorders.
Keywords: ErbB receptors; LTD; LTP; dopamine; hippocampus; midbrain dopamine neurons; neuregulins; synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wen D., Peles E., Cupples R., Suggs S.V., Bacus S.S., Luo Y., Trail G., Hu S., Silbiger S.M., Levy R.B., et al. Neu differentiation factor: A transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-M. - DOI - PubMed
-
- Brockes J.P., Lemke G.E., Balzer D.R., Jr. Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J. Biol. Chem. 1980;255:8374–8377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

