Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis
- PMID: 31906203
- PMCID: PMC7022468
- DOI: 10.3390/membranes10010007
Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis
Abstract
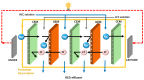
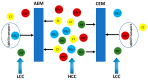
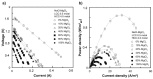
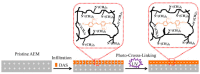
Reverse electrodialysis (RED) represents one of the most promising membrane-based technologies for clean and renewable energy production from mixing water solutions. However, the presence of multivalent ions in natural water drastically reduces system performance, in particular, the open-circuit voltage (OCV) and the output power. This effect is largely described by the "uphill transport" phenomenon, in which multivalent ions are transported against the concentration gradient. In this work, recent advances in the investigation of the impact of multivalent ions on power generation by RED are systematically reviewed along with possible strategies to overcome this challenge. In particular, the use of monovalent ion-selective membranes represents a promising alternative to reduce the negative impact of multivalent ions given the availability of low-cost materials and an easy route of membrane synthesis. A thorough assessment of the materials and methodologies used to prepare monovalent selective ion exchange membranes (both cation and anion exchange membranes) for applications in (reverse) electrodialysis is performed. Moreover, transport mechanisms under conditions of extreme salinity gradient are analyzed and compared for a better understanding of the design criteria. The ultimate goal of the present work is to propose a prospective research direction on the development of new membrane materials for effective implementation of RED under natural feed conditions.
Keywords: monovalent selective membranes; multivalent ions; reverse electrodialysis; salinity gradient power; uphill transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- BP BP Statstical Review of World Energy. [(accessed on 4 October 2019)]; Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdf....
-
- Tufa R.A., Curcio E., Fontananova E., Di Profio G. 3.8 Membrane-based processes for sustainable power generation using water: Pressure-retarded osmosis (PRO), reverse electrodialysis (RED), and capacitive mixing (CAPMIX) Compr. Membr. Sci. Eng. 2017;2:206–248.
-
- Tufa R.A., Pawlowski S., Veerman J., Bouzek K., Fontananova E., di Profio G., Velizarov S., Crespo J.G., Nijmeijer K., Curcio E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy. 2018;225:290–331. doi: 10.1016/j.apenergy.2018.04.111. - DOI
-
- Mei Y., Tang C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination. 2018;425:156–174. doi: 10.1016/j.desal.2017.10.021. - DOI
-
- Avci A.H., Sarkar P., Tufa R.A., Messana D., Argurio P., Fontananova E., Di Profio G., Curcio E. Effect of Mg2+ ions on energy generation by Reverse Electrodialysis. J. Membr. Sci. 2016;520:499–506. doi: 10.1016/j.memsci.2016.08.007. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

