Iron and Sphingolipids as Common Players of (Mal)Adaptation to Hypoxia in Pulmonary Diseases
- PMID: 31906427
- PMCID: PMC6981703
- DOI: 10.3390/ijms21010307
Iron and Sphingolipids as Common Players of (Mal)Adaptation to Hypoxia in Pulmonary Diseases
Abstract
Hypoxia, or lack of oxygen, can occur in both physiological (high altitude) and pathological conditions (respiratory diseases). In this narrative review, we introduce high altitude pulmonary edema (HAPE), acute respiratory distress syndrome (ARDS), Chronic Obstructive Pulmonary Disease (COPD), and Cystic Fibrosis (CF) as examples of maladaptation to hypoxia, and highlight some of the potential mechanisms influencing the prognosis of the affected patients. Among the specific pathways modulated in response to hypoxia, iron metabolism has been widely explored in recent years. Recent evidence emphasizes hepcidin as highly involved in the compensatory response to hypoxia in healthy subjects. A less investigated field in the adaptation to hypoxia is the sphingolipid (SPL) metabolism, especially through Ceramide and sphingosine 1 phosphate. Both individually and in concert, iron and SPL are active players of the (mal)adaptation to physiological hypoxia, which can result in the pathological HAPE. Our aim is to identify some pathways and/or markers involved in the physiological adaptation to low atmospheric pressures (high altitudes) that could be involved in pathological adaptation to hypoxia as it occurs in pulmonary inflammatory diseases. Hepcidin, Cer, S1P, and their interplay in hypoxia are raising growing interest both as prognostic factors and therapeutical targets.
Keywords: ARDS; COPD; Cystic Fibrosis; adaptation; ceramide; hepcidin; hypoxia; iron; sphingolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
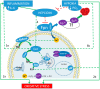
Similar articles
-
Increased hepcidin levels in high-altitude pulmonary edema.J Appl Physiol (1985). 2015 Feb 1;118(3):292-8. doi: 10.1152/japplphysiol.00940.2014. Epub 2014 Dec 18. J Appl Physiol (1985). 2015. PMID: 25525212
-
Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate.Prog Lipid Res. 2007 Mar;46(2):126-44. doi: 10.1016/j.plipres.2007.03.001. Epub 2007 Mar 14. Prog Lipid Res. 2007. PMID: 17449104 Review.
-
Susceptibility to high-altitude pulmonary edema is associated with circulating miRNA levels under hypobaric hypoxia conditions.Am J Physiol Lung Cell Mol Physiol. 2020 Aug 1;319(2):L360-L368. doi: 10.1152/ajplung.00168.2020. Epub 2020 Jun 17. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32692577
-
Sphingolipids in neurodegeneration (with focus on ceramide and S1P).Adv Biol Regul. 2018 Dec;70:51-64. doi: 10.1016/j.jbior.2018.09.013. Epub 2018 Sep 22. Adv Biol Regul. 2018. PMID: 30287225 Free PMC article. Review.
-
Sphingolipids as critical players in retinal physiology and pathology.J Lipid Res. 2021;62:100037. doi: 10.1194/jlr.TR120000972. Epub 2021 Feb 6. J Lipid Res. 2021. PMID: 32948663 Free PMC article. Review.
Cited by
-
SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression.Med Hypotheses. 2020 Oct;143:110102. doi: 10.1016/j.mehy.2020.110102. Epub 2020 Jul 13. Med Hypotheses. 2020. PMID: 32721799 Free PMC article.
-
Adaptation to Hypoxia: A Chimera?Int J Mol Sci. 2020 Feb 24;21(4):1527. doi: 10.3390/ijms21041527. Int J Mol Sci. 2020. PMID: 32102292 Free PMC article.
-
Alternative RAS in Various Hypoxic Conditions: From Myocardial Infarction to COVID-19.Int J Mol Sci. 2021 Nov 26;22(23):12800. doi: 10.3390/ijms222312800. Int J Mol Sci. 2021. PMID: 34884604 Free PMC article. Review.
-
Inside the Alterations of Circulating Metabolome in Antarctica: The Adaptation to Chronic Hypoxia.Front Physiol. 2022 Jan 25;13:819345. doi: 10.3389/fphys.2022.819345. eCollection 2022. Front Physiol. 2022. PMID: 35145434 Free PMC article.
-
Link between serum lipid signature and prognostic factors in COVID-19 patients.Sci Rep. 2021 Nov 4;11(1):21633. doi: 10.1038/s41598-021-00755-z. Sci Rep. 2021. PMID: 34737330 Free PMC article.
References
-
- Koskenkorva-Frank T.S., Weiss G., Koppenol W.H., Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. - DOI - PubMed
-
- Diab K.J., Adamowicz J.J., Kamocki K., Rush N.I., Garrison J., Gu Y., Schweitzer K.S., Skobeleva A., Rajashekhar G., Hubbard W.C., et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am. J. Respir. Crit. Care Med. 2010;181:344–352. doi: 10.1164/rccm.200906-0826OC. - DOI - PMC - PubMed
-
- Suresh M.V., Balijepalli S., Zhang B., Singh V.V., Swamy S., Panicker S., Dolgachev V.A., Subramanian C., Ramakrishnan S.K., Thomas B., et al. Hypoxia-Inducible Factor (HIF)-1alpha Promotes Inflammation and Injury Following Aspiration-Induced Lung Injury in Mice. Shock. 2019;52:612–621. doi: 10.1097/SHK.0000000000001312. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

