Can an Open-Label Placebo Be as Effective as a Deceptive Placebo? Methodological Considerations of a Study Protocol
- PMID: 31906435
- PMCID: PMC7168289
- DOI: 10.3390/medicines7010003
Can an Open-Label Placebo Be as Effective as a Deceptive Placebo? Methodological Considerations of a Study Protocol
Abstract
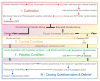
Background: Placebo has been studied for many years and is ever-present in healthcare. In clinical practice, its use is limited by ethical issues raised by the deception entailed by its administration. Objective: To investigate whether, when given detailed information about pain and underlying placebo mechanisms, subjects will have a response similar to that of those subjected to a procedure in which they receive a conventional placebo treatment. Methods: The study is designed as a non-inferiority randomized, parallel with a nested crossover trial. In addition, 126 subjects without any known pathology will be included. They will be randomized into two groups. Each subject will undergo three Cold Pressor Tests (CPT): calibration, condition of interest (deceptive placebo or educated placebo), and control. Our main judgment criterion will be the comparison in pain intensity experienced on the visual analog scale between the two CPTs with placebo conditions. Results: This study will allow us to rule on the non-inferiority of an "educated" placebo compared to a deceptive placebo in the context of an acute painful stimulation. It is another step towards the understanding of open-label placebo and its use in clinical practice. Conclusions: This study has been approved by the ethics committee in France (2017-A01643-50) and registered on ClinicalTrials.gov (NCT03934138).
Keywords: clinical trial; cold pressor test; ethics; open label placebo; pain; placebo.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
If only they knew! A non-inferiority randomized controlled trial comparing deceptive and open-label placebo in healthy individuals.Eur J Pain. 2024 Mar;28(3):491-501. doi: 10.1002/ejp.2204. Epub 2023 Nov 15. Eur J Pain. 2024. PMID: 37965922 Clinical Trial.
-
Deceptive but not open label placebos attenuate motion-induced nausea.J Psychosom Res. 2019 Oct;125:109808. doi: 10.1016/j.jpsychores.2019.109808. Epub 2019 Aug 12. J Psychosom Res. 2019. PMID: 31426018 Clinical Trial.
-
A comparison of open-label and deceptive placebo analgesia in a healthy sample.J Psychosom Res. 2021 Jan;140:110298. doi: 10.1016/j.jpsychores.2020.110298. Epub 2020 Nov 17. J Psychosom Res. 2021. PMID: 33227553
-
Placebos in the era of open-label trials: An update for clinicians.Eur J Clin Invest. 2019 Jan;49(1):e13038. doi: 10.1111/eci.13038. Epub 2018 Nov 1. Eur J Clin Invest. 2019. PMID: 30316203 Review.
-
Placebos as a Source of Agency: Evidence and Implications.Front Psychiatry. 2019 Oct 25;10:721. doi: 10.3389/fpsyt.2019.00721. eCollection 2019. Front Psychiatry. 2019. PMID: 31708807 Free PMC article. Review.
Cited by
-
Manual Therapy Effect in Placebo-Controlled Trials: A Systematic Review and Meta-Analysis.Int J Environ Res Public Health. 2022 Oct 28;19(21):14021. doi: 10.3390/ijerph192114021. Int J Environ Res Public Health. 2022. PMID: 36360901 Free PMC article.
-
Placebo: a brief updated review.Naunyn Schmiedebergs Arch Pharmacol. 2022 Nov;395(11):1343-1356. doi: 10.1007/s00210-022-02280-w. Epub 2022 Aug 9. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35943515 Free PMC article. Review.
References
-
- Kelley J.M. Lumping and Splitting: Toward a Taxonomy of Placebo and Related Effects. Int. Rev. Neurobiol. 2018;139:29–48. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical