Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD
- PMID: 31906479
- PMCID: PMC7016883
- DOI: 10.3390/cells9010110
Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD
Abstract
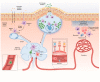
Infiltration of the lamina propria by inflammatory CD4+ T-cell populations is a key characteristic of chronic intestinal inflammation. Memory-phenotype CD4+ T-cell frequencies are increased in inflamed intestinal tissue of IBD patients compared to tissue of healthy controls and are associated with disease flares and a more complicated disease course. Therefore, a tightly controlled balance between regulatory and inflammatory CD4+ T-cell populations is crucial to prevent uncontrolled CD4+ T-cell responses and subsequent intestinal tissue damage. While at steady state, T-cells display mainly a regulatory phenotype, increased in Th1, Th2, Th9, Th17, and Th17.1 responses, and reduced Treg and Tr1 responses have all been suggested to play a role in IBD pathophysiology. However, it is highly unlikely that all these responses are altered in each individual patient. With the rapidly expanding plethora of therapeutic options to inhibit inflammatory T-cell responses and stimulate regulatory T-cell responses, a crucial need is emerging for a robust set of immunological assays to predict and monitor therapeutic success at an individual level. Consequently, it is crucial to differentiate dominant inflammatory and regulatory CD4+ T helper responses in patients and relate these to disease course and therapy response. In this review, we provide an overview of how intestinal CD4+ T-cell responses arise, discuss the main phenotypes of CD4+ T helper responses, and review how they are implicated in IBD.
Keywords: CD4+ T-cell; IBD; T helper cell; intestine.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
-
- Levine A., Griffiths A., Markowitz J., Wilson D.C., Turner D., Russell R.K., Fell J., Ruemmele F.M., Walters T., Sherlock M., et al. Pediatric Modification of the Montreal Classification for Inflammatory Bowel Disease: The Paris Classification. Inflamm. Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

