Polyethylene Glycol-Chitosan Oligosaccharide-Coated Superparamagnetic Iron Oxide Nanoparticles: A Novel Drug Delivery System for Curcumin Diglutaric Acid
- PMID: 31906490
- PMCID: PMC7023009
- DOI: 10.3390/biom10010073
Polyethylene Glycol-Chitosan Oligosaccharide-Coated Superparamagnetic Iron Oxide Nanoparticles: A Novel Drug Delivery System for Curcumin Diglutaric Acid
Abstract
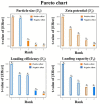
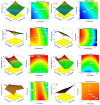
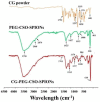
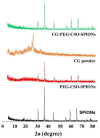
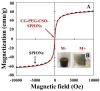
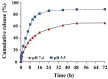
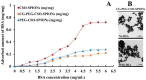
Curcumin diglutaric acid-loaded polyethylene glycol-chitosan oligosaccharide-coated superparamagnetic iron oxide nanoparticles (CG-PEG-CSO-SPIONs) were fabricated by co-precipitation and optimized using a Box-Behnken statistical design in order to achieve the minimum size, optimal zeta potential (≥ ±20 mV), and maximum loading efficiency and capacity. The results demonstrated that CG-PEG-CSO-SPIONs prepared under the optimal condition were almost spherical in shape with a smooth surface, a diameter of 130 nm, zeta potential of 30.6 mV, loading efficiency of 83.3%, and loading capacity of 8.3%. The vibrating sample magnetometer results of the optimized CG-PEG-CSO-SPIONs showed a superparamagnetic behavior. Fourier transform infrared spectroscopy and X-ray diffraction analyses indicated that the CG physically interacted with PEG-CSO-SPIONs. In addition, the CG-PEG-CSO-SPIONs could be stored dry for up to 12 weeks or in aqueous solution for up to 4 days at either 4 °C or 25 °C with no loss of stability. The CG-PEG-CSO-SPIONs exhibited a sustained release profile up to 72 h under simulated physiological (pH 7.4) and tumor extracellular (pH 5.5) environments. Furthermore, the CG-PEG-CSO-SPIONs showed little non-specific protein binding in the simulated physiological environment. The CG-PEG-CSO-SPIONs enhanced the cellular uptake and cytotoxicity of CG against human colorectal adenocarcinoma HT-29 cells compared to free CG, and more CG was delivered to the cells after applying an external magnetic field. The overall results suggest that PEG-CSO-SPIONs have potential to be used as a novel drug delivery system for CG.
Keywords: chitosan oligosaccharide; curcumin diglutaric acid; drug delivery systems; polyethylene glycol; superparamagnetic iron oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
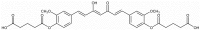





Similar articles
-
Preparation and characterization of chitosan-Polyethylene glycol-polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study.J Biomed Mater Res B Appl Biomater. 2016 May;104(4):808-16. doi: 10.1002/jbm.b.33637. Epub 2016 Mar 21. J Biomed Mater Res B Appl Biomater. 2016. PMID: 26996397
-
Release of a liver anticancer drug, sorafenib from its PVA/LDH- and PEG/LDH-coated iron oxide nanoparticles for drug delivery applications.Sci Rep. 2020 Dec 9;10(1):21521. doi: 10.1038/s41598-020-76504-5. Sci Rep. 2020. PMID: 33298980 Free PMC article.
-
Optimization, Characterization and in vivo Evaluation of Paclitaxel-Loaded Folate-Conjugated Superparamagnetic Iron Oxide Nanoparticles.Int J Nanomedicine. 2021 Mar 19;16:2283-2295. doi: 10.2147/IJN.S287434. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33776433 Free PMC article.
-
Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers.Int J Nanomedicine. 2012;7:3445-71. doi: 10.2147/IJN.S30320. Epub 2012 Jul 6. Int J Nanomedicine. 2012. PMID: 22848170 Free PMC article. Review.
-
Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications.Int J Nanomedicine. 2021 Aug 31;16:6097-6113. doi: 10.2147/IJN.S321984. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34511908 Free PMC article. Review.
Cited by
-
Fabrication of Curcumin Diethyl γ-Aminobutyrate-Loaded Chitosan-Coated Magnetic Nanocarriers for Improvement of Cytotoxicity against Breast Cancer Cells.Polymers (Basel). 2022 Dec 19;14(24):5563. doi: 10.3390/polym14245563. Polymers (Basel). 2022. PMID: 36559930 Free PMC article.
-
Mucoadhesive carriers for oral drug delivery.J Control Release. 2022 Nov;351:504-559. doi: 10.1016/j.jconrel.2022.09.024. Epub 2022 Sep 30. J Control Release. 2022. PMID: 36116580 Free PMC article. Review.
-
Electrospun Core-Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance.Biomolecules. 2022 Jul 29;12(8):1057. doi: 10.3390/biom12081057. Biomolecules. 2022. PMID: 36008951 Free PMC article.
-
In Vivo Distribution of Poly(ethylene glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study.Nanomaterials (Basel). 2021 Aug 25;11(9):2184. doi: 10.3390/nano11092184. Nanomaterials (Basel). 2021. PMID: 34578500 Free PMC article.
-
Curcumin Nanoformulations with Metal Oxide Nanomaterials for Biomedical Applications.Nanomaterials (Basel). 2021 Feb 11;11(2):460. doi: 10.3390/nano11020460. Nanomaterials (Basel). 2021. PMID: 33670161 Free PMC article. Review.
References
-
- Miranda M.S., Rodrigues M.T., Domingues R.M.A., Costa R.R., Paz E., Rodríguez-Abreu C., Freitas P., Almeida B.G., Carvalho M.A., Gonçalves C., et al. Development of inhalable superparamagnetic iron oxide nanoparticles (SPIONs) in microparticulate system for antituberculosis drug delivery. Adv. Healthc. Mater. 2018;7:1800124. - PubMed
-
- Satari M., Haghighat N., Jouni F.J., Abodolmaleki P. Effects of synthesized superparamagnetic iron oxide nanoparticles and extremely low frequency. Multidiscip. Cancer Investig. 2018;2:13–21. doi: 10.30699/acadpub.mci.2.1.13. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

