Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants
- PMID: 31906503
- PMCID: PMC7023585
- DOI: 10.3390/polym12010066
Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants
Abstract
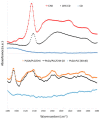
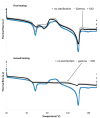
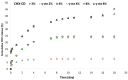
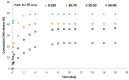
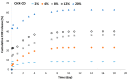
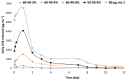
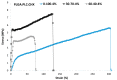
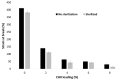
To prevent the uncontrolled development of a pathogenic biofilm around a dental implant, an antimicrobial drug-release electrospun membrane, set up between the implant and the gingival tissue, was developed by taking several technical, industrial and regulatory specifications into account. The membrane formulation is made of a blend of poly(l-lactic-co-gycolic acid) (PLGA, 85:15) and poly(l-lactic acide-co-ɛ-caprolactone) (PLC, 70:30) copolymers with chlorhexidine diacetate (CHX) complexed with β-cyclodextrin (CD). The amount of residual solvent, the mechanical properties and the drug release kinetics were tuned by the copolymers' ratio, between 30% and 100% of PLC, and a CHX loading up to 20% w/w. The membranes were sterilized by γ-irradiation without significant property changes. The fiber's diameter was between 600 nm and 3 µm, depending on the membrane composition and the electrospinning parameters. CHX was released in vitro over 10 days and the bacterial inhibitory concentration, 80 µg·mL-1, was reached within eight days. The optimal membrane, PGLA/PLC/CHX-CD (60%/40%/4%), exhibited a breaking strain of 50%, allowing its safe handling. This membrane and a membrane without CHX-CD were implanted subcutaneous in a rat model. The cell penetration remained low. The next step will be to increase the porosity of the membrane to improve the dynamic cell penetration and tissue remodeling.
Keywords: bioresorbable polymers; dental membrane; drug delivery; electrospinning; peri-implantitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes.Polymers (Basel). 2021 Feb 25;13(5):695. doi: 10.3390/polym13050695. Polymers (Basel). 2021. PMID: 33669032 Free PMC article.
-
A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease.Biomaterials. 2004 Aug;25(17):3743-50. doi: 10.1016/j.biomaterials.2003.09.113. Biomaterials. 2004. PMID: 15020150
-
Does chlorhexidine improve outcomes in non-surgical management of peri-implant mucositis or peri-implantitis?: a systematic review and meta-analysis.Med Oral Patol Oral Cir Bucal. 2020 Sep 1;25(5):e608-e615. doi: 10.4317/medoral.23633. Med Oral Patol Oral Cir Bucal. 2020. PMID: 32683389 Free PMC article.
-
Chlorhexidine Uptake and Release From Modified Titanium Surfaces and Its Antimicrobial Activity.J Periodontol. 2015 Nov;86(11):1268-75. doi: 10.1902/jop.2015.150075. Epub 2015 Jul 9. J Periodontol. 2015. PMID: 26156675
-
Efficacy of chlorhexidine rinses after periodontal or implant surgery: a systematic review.Clin Oral Investig. 2019 Jan;23(1):21-32. doi: 10.1007/s00784-018-2761-y. Epub 2018 Dec 7. Clin Oral Investig. 2019. PMID: 30535817
Cited by
-
Electrospun nanofibers: Focus on local therapeutic delivery targeting infectious disease.J Drug Deliv Sci Technol. 2025 Feb;104:106520. doi: 10.1016/j.jddst.2024.106520. Epub 2024 Dec 20. J Drug Deliv Sci Technol. 2025. PMID: 39802685
-
Study of PVP and PLA Systems and Fibers Obtained by Solution Blow Spinning for Chlorhexidine Release.Polymers (Basel). 2025 Jun 30;17(13):1839. doi: 10.3390/polym17131839. Polymers (Basel). 2025. PMID: 40647849 Free PMC article.
-
The Effect of Microcosm Biofilm Decontamination on Surface Topography, Chemistry, and Biocompatibility Dynamics of Implant Titanium Surfaces.Int J Mol Sci. 2022 Sep 2;23(17):10033. doi: 10.3390/ijms231710033. Int J Mol Sci. 2022. PMID: 36077428 Free PMC article.
-
Microwave-Assisted Fabrication of Mesoporous Silica-Calcium Phosphate Composites for Dental Application.Polymers (Basel). 2020 Dec 25;13(1):53. doi: 10.3390/polym13010053. Polymers (Basel). 2020. PMID: 33375650 Free PMC article.
-
Polycaprolactone Electrospun Nanofiber Membrane with Sustained Chlorohexidine Release Capability against Oral Pathogens.J Funct Biomater. 2022 Dec 7;13(4):280. doi: 10.3390/jfb13040280. J Funct Biomater. 2022. PMID: 36547540 Free PMC article.
References
-
- Buser D., Janner S.F.M., Wittneben J.-G., Brägger U., Ramseier C.A., Salvi G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012;14:839–851. doi: 10.1111/j.1708-8208.2012.00456.x. - DOI - PubMed
-
- van Velzen F.J.J., Ofec R., Schulten E.A.J.M., Ten Bruggenkate C.M. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral Implants Res. 2015;26:1121–1128. doi: 10.1111/clr.12499. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

