Role of FAD-I in Fusobacterial Interspecies Interaction and Biofilm Formation
- PMID: 31906541
- PMCID: PMC7023056
- DOI: 10.3390/microorganisms8010070
Role of FAD-I in Fusobacterial Interspecies Interaction and Biofilm Formation
Erratum in
-
Correction: Shokeen, B., et al. Role of FAD-I in Fusobacterial Interspecies Interaction and Biofilm Formation. Microorganisms 2020, 8, 70.Microorganisms. 2020 Dec 29;9(1):63. doi: 10.3390/microorganisms9010063. Microorganisms. 2020. PMID: 33383978 Free PMC article.
Abstract
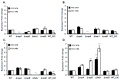
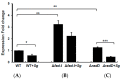
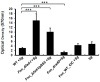
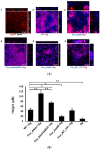
: RadD, a major adhesin of oral fusobacteria, is part of a four-gene operon encoding the small lipoprotein FAD-I and two currently uncharacterized small proteins encoded by the rapA and rapB genes. Previously, we described a role for FAD-I in the induction of human B-defensin 2 (hBD2) upon contact with oral epithelial cells. Here, we investigated potential roles for fad-I, rapA, and rapB in interspecies interaction and biofilm formation. Gene inactivation mutants were generated for each of these genes in the nucleatum and polymorphum subspecies of Fusobacterium nucleatum and characterized for their adherence to partner species, biofilm formation, and operon transcription. Binding to Streptococcus gordonii was increased in all mutant strains with Δfad-I having the most significant effect. This increased adherence was directly proportional to elevated radD transcript levels and resulted in significantly different architecture and height of the biofilms formed by Δfad-I and S. gordonii compared to the wild-type parent. In conclusion, FAD-I is important for fusobacterial interspecies interaction as its lack leads to increased production of the RadD adhesin suggesting a role of FAD-I in its regulation. This regulatory effect does not require the presence of functional RadD.
Keywords: Fusobacterium nucleatum; RadD; biofilm; fad-I; interspecies interaction.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




References
-
- Handley P., Rickard A. Coaggregation—Is it a universal biofilm phenomenon? In: Gilbert P., Allison D., editors. Biofilm Community Interactions: Chance or Necessity? Bioline; London, UK: 2001. pp. 1–10.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

