Enzyme Activity Profiles Produced on Wood and Straw by Four Fungi of Different Decay Strategies
- PMID: 31906600
- PMCID: PMC7022816
- DOI: 10.3390/microorganisms8010073
Enzyme Activity Profiles Produced on Wood and Straw by Four Fungi of Different Decay Strategies
Abstract
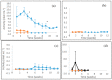
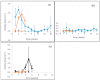
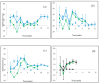
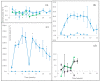
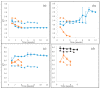
Four well-studied saprotrophic Basidiomycota Agaricomycetes species with different decay strategies were cultivated on solid lignocellulose substrates to compare their extracellular decomposing carbohydrate-active and lignin-attacking enzyme production profiles. Two Polyporales species, the white rot fungus Phlebia radiata and brown rot fungus Fomitopsis pinicola, as well as one Agaricales species, the intermediate "grey" rot fungus Schizophyllum commune, were cultivated on birch wood pieces for 12 weeks, whereas the second Agaricales species, the litter-decomposing fungus Coprinopsis cinerea was cultivated on barley straw for 6 weeks under laboratory conditions. During 3 months of growth on birch wood, only the white rot fungus P. radiata produced high laccase and MnP activities. The brown rot fungus F. pinicola demonstrated notable production of xylanase activity up to 43 nkat/mL on birch wood, together with moderate β-glucosidase and endoglucanase cellulolytic activities. The intermediate rot fungus S. commune was the strongest producer of β-glucosidase with activities up to 54 nkat/mL, and a notable producer of xylanase activity, even up to 620 nkat/mL, on birch wood. Low lignin-attacking but moderate activities against cellulose and hemicellulose were observed with the litter-decomposer C. cinerea on barley straw. Overall, our results imply that plant cell wall decomposition ability of taxonomically and ecologically divergent fungi is in line with their enzymatic decay strategy, which is fundamental in understanding their physiology and potential for biotechnological applications.
Keywords: Basidiomycota; biodegradation; carbohydrate active enzymes; enzyme activity assays; laccase; lignocellulose; manganese peroxidase; organic acids; oxalic acid; wood decay fungi.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Lundell T.K., Mäkelä M.R., de Vries R.P., Hildén K.S. Genomics, lifestyles and future prospects of wood-decay and litter-decomposing Basidiomycota. Adv. Bot. Res. 2014;70:329–370. doi: 10.1016/B978-0-12-397940-7.00011-2. - DOI
-
- Hatakka A., Hammel K.E. Fungal biodegradation of lignocelluloses. In: Esser K., Hofrichter M., editors. The Mycota. Industrial Applications. Volume 10. Springer; Berlin, Germany: 2010. pp. 319–340.
-
- Floudas D., Binder M., Riley R., Barry K., Blanchette R.A., Henrissat B., Martinez A.T., Otillar R., Spatafora J.W., Yadav J.S., et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336:1715–1719. doi: 10.1126/science.1221748. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

