Vitamin D supplementation compared to placebo in people with First Episode psychosis - Neuroprotection Design (DFEND): a protocol for a randomised, double-blind, placebo-controlled, parallel-group trial
- PMID: 31907006
- PMCID: PMC6945550
- DOI: 10.1186/s13063-019-3758-9
Vitamin D supplementation compared to placebo in people with First Episode psychosis - Neuroprotection Design (DFEND): a protocol for a randomised, double-blind, placebo-controlled, parallel-group trial
Abstract
Background: People experiencing their first episode of psychosis are often deficient in vitamin D. Observational studies have reported an association between low vitamin D concentrations and poorer subsequent health outcomes in psychosis. A vitamin D deficiency in neonates and children has been linked to a later increased risk of schizophrenia and psychotic-like experiences. This trial aims to examine the effect of high-dose vitamin D supplementation on outcomes in early psychosis. We hypothesise that vitamin D supplementation will be associated with better mental health outcomes.
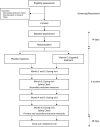
Methods/design: The DFEND study is a multicentre double-blind placebo-controlled parallel-group trial of vitamin D supplementation in people with early psychosis. Patients with an ICD-10 diagnosis of functional psychosis will be randomised in a 1:1 ratio to receive either 120,000 IU/month of vitamin D (cholecalciferol) or a matched placebo for 6 months. The primary outcome is the total Positive and Negative Syndrome Scale (PANSS) score at the 6-month follow-up for all patients. Secondary outcomes include assessment of mood (Calgary Depression Scale), general function (Global Assessment of Functioning), cardiovascular risk (body mass index, waist circumference, C-reactive protein, cholesterol and HbA1c) and vitamin D levels at the 6-month follow-up. Additionally, 3- and 6-month total PANSS scores will be analysed for those with inadequate vitamin D levels at the baseline.
Discussion: The DFEND study is the first trial to examine whether vitamin D supplementation in early psychosis is associated with better mental health outcomes. The findings of this study may help to resolve the clinical equipoise regarding the benefits and cost-effectiveness of routine vitamin D supplementation in people with psychosis.
Trial registration: ISRCTN, ISRCTN12424842. Registered on 25 February 2015.
Keywords: 25OHD; First episode; Mental health; Positive and Negative Syndrome Scale; Psychosis; Randomised controlled trial; Vitamin D.
Conflict of interest statement
FG has received honoraria for advisory work and lectures, has received support for Continuing Medical Education (CME) activities in the last 3 years from Lundbeck, Otsaka and Sunovion, is a collaborator on an NHS Innovations project co-funded by Janssen and has a family member with professional links to Lilly and GSK, including shares. RM has received honoraria for lectures from Lundbeck, Janssen and Sunovion. SR has received honoraria for lectures from Lundbeck. All other authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

