Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study)
- PMID: 31907007
- PMCID: PMC6945422
- DOI: 10.1186/s13063-019-3734-4
Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study)
Abstract
Background: Infliximab (INX) and other tumour necrosis factor inhibitors (TNFi) have revolutionised the treatment of several immune mediated inflammatory diseases. Still, many patients do not respond sufficiently to therapy or lose efficacy over time. The large interindividual variation in serum drug concentrations on standard doses and the development of anti-drug antibodies are thought to be major reasons for treatment failures. Therapeutic drug monitoring (TDM), an individualised treatment strategy based on systematic assessments of serum drug concentrations, has been proposed as a clinical tool to optimise efficacy of INX treatment. TDM seems reasonable both from a clinical and an economical point of view, but the effectiveness of this treatment strategy has not yet been demonstrated in randomised clinical trials. The NORwegian DRUg Monitoring study (NOR-DRUM) aims to assess the effectiveness of TDM, both with regard to the achievement of remission in patients starting INX treatment (part A) as well as to maintain disease control in patients on INX treatment (part B).
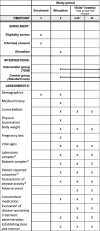
Methods: The NOR-DRUM study is a randomised, open, controlled, parallel-group, comparative, multi-centre, national, superiority, phase IV study with two separate parts, NOR-DRUM A and NOR-DRUM B. Patients with rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, ulcerative colitis, Crohn's disease and psoriasis are included. In both study parts participants are randomised 1:1 to either TDM of infliximab (intervention group) or to standard treatment with infliximab without knowledge of drug levels or ADAb status (control group). NOR-DRUM A will include 400 patients starting INX therapy. The primary outcome is remission at 30 weeks. In NOR-DRUM B, 450 patients on maintenance treatment with INX will be included. The primary endpoint is occurrence of disease worsening during the 52-week study period.
Discussion: As the first trial to assess the effectiveness, safety and cost-effectiveness of TDM in patients receiving TNFi for a range of immune mediated inflammatory diseases, we hope that the NOR-DRUM study will contribute to the advancement of evidence based personalised treatment with biological medicines.
Trial registration: Clinicaltrials.gov, NCT03074656. Registered on 090317.
Keywords: Immunogenicity; Infliximab; Personalised medicine; Serum drug levels; Therapeutic drug monitoring.
Conflict of interest statement
SWS reports personal fees for speaking from Roche.
GLG reports personal fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sandoz, Orion Pharma, Celltrion and Boehringer Ingelheim.
KKJ reports personal fees from Intercept, Norgine and Celltrion.
ICJ reports personal fees from Pfizer.
ØS declares that he has no competing interests.
JG reports personal fees for speaking from Roche.
DJW declares that he has no competing interests.
JS declares that he has no competing interests.
CM reports fees for speaking and/or consulting from Novartis, LEO Pharma, ACO, Petrified Nordic, Abbvie, Galderma Nordic, Celgene.
TKK reports fees for speaking and/or consulting from AbbVie, Biogen, Celltrion, Egis, Eli Lilly, Hikma, MSD, Mylan, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Roche, Sandoz, Sanofi and UCB and has received research funding to Diakonhjemmet Hospital from AbbVie, BMS, MSD, Pfizer, Roche and UCB.
JJ has served as a speaker, consultant or advisory board member for AbbVie, Astro Pharma, Boerhinger Ingelheim, BMS, Celltrion, Ferring, Hikma, Janssen, Meda, MSD, Napp Pharma, Norgine, Novartis, Orion Pharma, Pfizer, Pharmacosmos, Roche, Takeda, Tillotts and Sandoz.
NB reports personal fees from Orion Pharma, Roche, Napp Pharmaceuticals, Pfizer and Takeda.
EAH reports fees for speaking and/or consulting from AbbVie, Eli Lilly, Pfizer, Roche, Celgene, Janssen and UCB and has received research funding to Diakonhjemmet Hospital from AbbVie, MSD, Pfizer, Roche and UCB.
Figures




References
-
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
-
- Braun J, Landewe R, Hermann KG, Han J, Yan S, Williamson P, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum. 2006;54(5):1646–1652. doi: 10.1002/art.21790. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

