Effect and associated factors of a clinical pharmacy model in the incidence of medication errors (EACPharModel) in the Hospital Pablo Tobón Uribe: study protocol for a stepped wedge randomized controlled trial (NCT03338725)
- PMID: 31907009
- PMCID: PMC6945697
- DOI: 10.1186/s13063-019-3945-8
Effect and associated factors of a clinical pharmacy model in the incidence of medication errors (EACPharModel) in the Hospital Pablo Tobón Uribe: study protocol for a stepped wedge randomized controlled trial (NCT03338725)
Abstract
Background: According to WHO, medication error (ME) is a subject that requires attention at all levels of care to reduce severe and preventable damage related to medication use. Clinical pharmacy practice standards have been proposed around the world so that the pharmacist, as part of a multidisciplinary health team, can help improve patient safety; however, further evidence derived from adequate studies is needed to demonstrate this. This study aims to assess the effect of a clinical pharmacy practice model (CPPM) in preventing MEs associated with the medication use process.
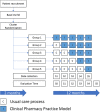
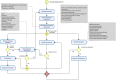
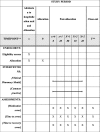
Methods: A prospective, stepped-wedge, cluster-randomized, controlled trial with a duration of 14 months will be performed to compare the effect of a CPPM along with the usual care process of patients in the Pablo Tobón Uribe Hospital (Medellin, Colombia). The study is designed as a cluster-randomized controlled trial, involving five hospital wards (clusters) and 720 patients. Medical wards are allocated to interventions using a stepped-wedge design. Clusters are initially assigned to the control group. After a 2-month observation period, hospital clusters were randomly allocated to the intervention group. Study outcomes will be assessed at baseline and at 2, 4, 6, 8, 10, and 12 months after randomization. The primary outcome will be to assess the effect of a CPPM on the incidence of medication errors associated with the medication use process. Drug-related problems and factors that contribute to the occurrence of MEs will be assessed as secondary outcomes. Statistical analyses will be performed using a mixed model, with the treatment group and time as fixed effects and the clustering structure as a random effect. Statistical analysis will be performed using Pearson chi-square tests and Student's t-tests, and a P value < 0.05 will be considered statistically significant.
Discussion: As far as we know, this is the first stepped-wedge, cluster-randomized, controlled trial designed to assess the change of a CPPM on the incidence of medication errors in a hospital in Colombia, and it could generate valuable information about a standardized and patient-centered clinical pharmacy model to improve the safety of inpatient care.
Trial registration: ClinicalTrials.gov, NCT03338725. Registered on 9 November 2017. The first patient was randomized on 2 February 2018.
Protocol version: 0010112018JG.
Keywords: Medication errors, Drug-related problems, Pharmacy service, Hospital (clinical pharmacy services), Pharmacists.
Conflict of interest statement
The authors declare they do not have any potential conflicts of interest concerning the authorship or publication of this article. Data are being collected by JGV and will be interpreted by JGV, PA, JPD, NO, and ASO. JGV, PA, JBD, and ASO will write the paper.
Figures

References
-
- Kohn LT, Corrigan J, Donaldson MS, editors. To err is human: building a safer health system. Washington, D.C.: National Academy Press; 2000. - PubMed
-
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 353:i2139. 10.1136/bmj.i2139. - PubMed
-
- McLeod M, Barber N, Franklin BD. Facilitators and barriers to safe medication administration to hospital inpatients: a mixed methods study of nurses’ Medication Administration Processes and Systems (the MAPS Study) PLoS. One. 2015;10(6):e0128958. doi: 10.1371/journal.pone.0128958. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

