Derivatives of the Antimalarial Drug Mefloquine Are Broad-Spectrum Antifungal Molecules with Activity against Drug-Resistant Clinical Isolates
- PMID: 31907188
- PMCID: PMC7038245
- DOI: 10.1128/AAC.02331-19
Derivatives of the Antimalarial Drug Mefloquine Are Broad-Spectrum Antifungal Molecules with Activity against Drug-Resistant Clinical Isolates
Abstract
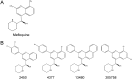
The antifungal pharmacopeia is critically small, particularly in light of the recent emergence of multidrug-resistant pathogens, such as Candida auris Here, we report that derivatives of the antimalarial drug mefloquine have broad-spectrum antifungal activity against pathogenic yeasts and molds. In addition, the mefloquine derivatives have activity against clinical isolates that are resistant to one or more of the three classes of antifungal drugs currently used to treat invasive fungal infections, indicating that they have a novel mechanism of action. Importantly, the in vitro toxicity profiles obtained using human cell lines indicated that the toxicity profiles of the mefloquine derivatives are very similar to those of the parent mefloquine, despite being up to 64-fold more active against fungal cells. In addition to direct antifungal activity, subinhibitory concentrations of the mefloquine derivatives inhibited the expression of virulence traits, including filamentation in Candida albicans and capsule formation/melanization in Cryptococcus neoformans Mode/mechanism-of-action experiments indicated that the mefloquine derivatives interfere with both mitochondrial and vacuolar function as part of a multitarget mechanism of action. The broad-spectrum scope of activity, blood-brain barrier penetration, and large number of previously synthesized analogs available combine to support the further optimization and development of the antifungal activity of this general class of drug-like molecules.
Keywords: Aspergillus; Candida; Candida auris; Cryptococcus; Cryptococcus neoformans; antifungal; antifungal agents; mefloquine; repurposing.
Copyright © 2020 American Society for Microbiology.
Figures






Similar articles
-
Analogs of the anti-malaria drug mefloquine have broad-spectrum antifungal activity and are efficacious in a model of disseminated Candida auris infection.Antimicrob Agents Chemother. 2024 Nov 6;68(11):e0130124. doi: 10.1128/aac.01301-24. Epub 2024 Oct 4. Antimicrob Agents Chemother. 2024. PMID: 39365066 Free PMC article.
-
In Vitro Activity of APX001A (Manogepix) and Comparator Agents against 1,706 Fungal Isolates Collected during an International Surveillance Program in 2017.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00840-19. doi: 10.1128/AAC.00840-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31182527 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Alexidine Dihydrochloride Has Broad-Spectrum Activities against Diverse Fungal Pathogens.mSphere. 2018 Oct 31;3(5):e00539-18. doi: 10.1128/mSphere.00539-18. mSphere. 2018. PMID: 30381356 Free PMC article.
-
Antifungal drug resistance in pathogenic fungi.Med Mycol. 1998;36 Suppl 1:119-28. Med Mycol. 1998. PMID: 9988500 Review.
Cited by
-
Analogues of the anti-malaria drug mefloquine have broad spectrum antifungal activity and are efficacious in a model of disseminated Candida auris infection.bioRxiv [Preprint]. 2024 Aug 30:2024.08.29.610379. doi: 10.1101/2024.08.29.610379. bioRxiv. 2024. Update in: Antimicrob Agents Chemother. 2024 Nov 6;68(11):e0130124. doi: 10.1128/aac.01301-24. PMID: 39257751 Free PMC article. Updated. Preprint.
-
The GPCR antagonist PPTN synergizes with caspofungin providing increased fungicidal activity against Aspergillus fumigatus.Microbiol Spectr. 2025 Mar 17;13(5):e0331824. doi: 10.1128/spectrum.03318-24. Online ahead of print. Microbiol Spectr. 2025. PMID: 40090930 Free PMC article.
-
Screening and transcriptomic analysis of anti-Sporothrix globosa targeting AbaA.Front Microbiol. 2025 Apr 29;16:1546020. doi: 10.3389/fmicb.2025.1546020. eCollection 2025. Front Microbiol. 2025. PMID: 40365064 Free PMC article.
-
Recent Progress in Research on Mitochondrion-Targeted Antifungal Drugs: a Review.Antimicrob Agents Chemother. 2023 Jun 15;67(6):e0000323. doi: 10.1128/aac.00003-23. Epub 2023 May 17. Antimicrob Agents Chemother. 2023. PMID: 37195189 Free PMC article. Review.
-
Progress in the study of mefloquine as an antibiotic adjuvant for combination bacterial inhibition treatment.Front Cell Infect Microbiol. 2024 Nov 28;14:1470891. doi: 10.3389/fcimb.2024.1470891. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39669268 Free PMC article. Review.
References
-
- Chen Y, Farrer RA, Giamberardino C, Sakthikumar S, Jones A, Yang T, Tenor JL, Wagih O, Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP, Cuomo CA, Perfect JR. 2017. Microevolution of serial clinical isolates of Cryptococcus neoformans var. grubii and C. gattii. mBio 8:e00166-17. doi:10.1128/mBio.00166-17. - DOI - PMC - PubMed
-
- Rhein J, Huppler Hullsiek K, Tugume L, Nuwagira E, Mpoza E, Evans EE, Kiggundu R, Pastick KA, Ssebambulidde K, Akampurira A, Williams DA, Bangdiwala AS, Abassi M, Musubire AK, Nicol MR, Muzoora C, Meya DB, Boulware DR, ASTRO-CM Team. 2019. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 19:843–851. doi:10.1016/S1473-3099(19)30127-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

