Brain Parenchymal and Extraparenchymal Macrophages in Development, Homeostasis, and Disease
- PMID: 31907272
- PMCID: PMC7034672
- DOI: 10.4049/jimmunol.1900821
Brain Parenchymal and Extraparenchymal Macrophages in Development, Homeostasis, and Disease
Abstract
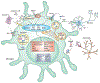
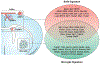
Microglia are parenchymal macrophages of the CNS; as professional phagocytes they are important for maintenance of the brain's physiology. These cells are generated through primitive hematopoiesis in the yolk sac and migrate into the brain rudiment after establishment of embryonic circulation. Thereafter, microglia develop in a stepwise fashion, reaching complete maturity after birth. In the CNS, microglia self-renew without input from blood monocytes. Recent RNA-sequencing studies have defined a molecular signature for microglia under homeostasis. However, during disease, microglia undergo remarkable phenotypic changes, which reflect the acquisition of specialized functions tailored to the pathological context. In addition to microglia, the brain-border regions host populations of extraparenchymal macrophages with disparate origins and phenotypes that have recently been delineated. In this review we outline recent findings that provide a deeper understanding of both parenchymal microglia and extraparenchymal brain macrophages in homeostasis and during disease.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures


References
-
- Sieweke MH, and Allen JE. 2013. Beyond stem cells: self-renewal of differentiated macrophages. Science 342: 1242974. - PubMed
-
- Ginhoux F, and Jung S. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol 14: 392–404. - PubMed
-
- Ginhoux F, and Guilliams M. 2016. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 44: 439–449. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

