Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks
- PMID: 31907460
- PMCID: PMC6960329
- DOI: 10.1038/s41591-019-0715-9
Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks
Abstract
Intraoperative diagnosis is essential for providing safe and effective care during cancer surgery1. The existing workflow for intraoperative diagnosis based on hematoxylin and eosin staining of processed tissue is time, resource and labor intensive2,3. Moreover, interpretation of intraoperative histologic images is dependent on a contracting, unevenly distributed, pathology workforce4. In the present study, we report a parallel workflow that combines stimulated Raman histology (SRH)5-7, a label-free optical imaging method and deep convolutional neural networks (CNNs) to predict diagnosis at the bedside in near real-time in an automated fashion. Specifically, our CNNs, trained on over 2.5 million SRH images, predict brain tumor diagnosis in the operating room in under 150 s, an order of magnitude faster than conventional techniques (for example, 20-30 min)2. In a multicenter, prospective clinical trial (n = 278), we demonstrated that CNN-based diagnosis of SRH images was noninferior to pathologist-based interpretation of conventional histologic images (overall accuracy, 94.6% versus 93.9%). Our CNNs learned a hierarchy of recognizable histologic feature representations to classify the major histopathologic classes of brain tumors. In addition, we implemented a semantic segmentation method to identify tumor-infiltrated diagnostic regions within SRH images. These results demonstrate how intraoperative cancer diagnosis can be streamlined, creating a complementary pathway for tissue diagnosis that is independent of a traditional pathology laboratory.
Conflict of interest statement
Figures












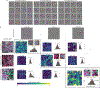
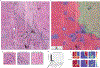
Comment in
-
Intraoperative brain tumour identification with deep learning.Nat Rev Clin Oncol. 2020 Apr;17(4):200-201. doi: 10.1038/s41571-020-0343-9. Nat Rev Clin Oncol. 2020. PMID: 32099093 No abstract available.
References
-
- Sullivan R et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 16, 1193–1224 (2015). - PubMed
-
- Novis DA & Zarbo RJ Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-Probes study of 32868 frozen sections in 700 hospitals. Arch. Pathol. Lab. Med 121, 559–567 (1997). - PubMed
-
- Gal AA & Cagle PT The 100-year anniversary of the description of the frozen section procedure. JAMA 294, 3135–3137 (2005). - PubMed
-
- Robboy SJ et al. Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Arch. Pathol. Lab. Med 137, 1723–1732 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

