Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy
- PMID: 31907599
- PMCID: PMC8179912
- DOI: 10.1007/s00415-019-09688-0
Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy
Abstract
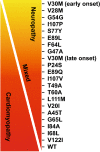
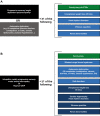
Amyloid transthyretin (ATTR) amyloidosis with polyneuropathy (PN) is a progressive, debilitating, systemic disease wherein transthyretin protein misfolds to form amyloid, which is deposited in the endoneurium. ATTR amyloidosis with PN is the most serious hereditary polyneuropathy of adult onset. It arises from a hereditary mutation in the TTR gene and may involve the heart as well as other organs. It is critical to identify and diagnose the disease earlier because treatments are available to help slow the progression of neuropathy. Early diagnosis is complicated, however, because presentation may vary and family history is not always known. Symptoms may be mistakenly attributed to other diseases such as chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), idiopathic axonal polyneuropathy, lumbar spinal stenosis, and, more rarely, diabetic neuropathy and AL amyloidosis. In endemic countries (e.g., Portugal, Japan, Sweden, Brazil), ATTR amyloidosis with PN should be suspected in any patient who has length-dependent small-fiber PN with autonomic dysfunction and a family history of ATTR amyloidosis, unexplained weight loss, heart rhythm disorders, vitreous opacities, or renal abnormalities. In nonendemic countries, the disease may present as idiopathic rapidly progressive sensory motor axonal neuropathy or atypical CIDP with any of the above symptoms or with bilateral carpal tunnel syndrome, gait disorders, or cardiac hypertrophy. Diagnosis should include DNA testing, biopsy, and amyloid typing. Patients should be followed up every 6-12 months, depending on the severity of the disease and response to therapy. This review outlines detailed recommendations to improve the diagnosis of ATTR amyloidosis with PN.
Keywords: ATTR amyloidosis; ATTRv; Diagnosis; Peripheral neuropathy; Transthyretin amyloidosis; hATTR.
Conflict of interest statement
This review was sponsored by the Amyloidosis Research Consortium. Dr Adams reports grants from Alnylam and Pfizer and personal fees from Prothena, GSK, Alnylam, and Pfizer. Dr Ando has nothing to disclose. Dr Beirão has nothing to disclose. Dr Coelho was paid per protocol for clinical trials from FoldRx, Pfizer, Ionis, and Alnylam and received grants from FoldRx and Pfizer; received support from Pfizer, Ionis, Biogen, and Alnylam to attend scientific meetings; and has presented on behalf of Pfizer, Alnylam, GSK, Prothena, and Ionis/Akcea and received honoraria. Dr Gertz has received personal fees from Ionis/Akcea, Alnylam, Prothena, Celgene, Janssen, Annexon, Appellis, Amgen, Medscape, Physicians Education Resource, and Research to Practice; grants and personal fees from Spectrum; personal fees for Data Safety Monitoring board from AbbVie; speaker fees from Teva, Johnson and Johnson, Medscape, and DAVA Oncology; royalties from Springer Publishing; and grant funding from the Amyloidosis Foundation, the International Waldenström Foundation, and the National Cancer Institute (SPORE MM SPORE 5P50 CA186781-04). In addition, he has served on advisory boards for Pharmacyclics and for Proclara (outside the submitted work). Dr Gillmore has participated in advisory boards for Alnylam Pharmaceuticals Inc and GSK Inc. Dr Hawkins has nothing to disclose. Ms Lousada has received honoraria from Akcea. Dr Suhr has received honoraria and travel and consultancy fees from Ionis/Akcea, Alnylam, Prothena, and Intellia. Dr Merlini has received honoraria from Janssen and Prothena; travel support from Prothena and Celgene; and consulting fees from Millennium, Pfizer, Janssen, Prothena, and Ionis.
Figures

References
-
- Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15(7):387–404. - PubMed
-
- Rapezzi C, Quarta CC, Obici L, Perfetto F, Longhi S, Salvi F, Biagini E, Lorenzini M, Grigioni F, Leone O, Cappelli F, Palladini G, Rimessi P, Ferlini A, Arpesella G, Pinna AD, Merlini G, Perlini S. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34:520–528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

