Efficient recruitment of c-FLIPL to the death-inducing signaling complex leads to Fas resistance in natural killer-cell lymphoma
- PMID: 31908105
- PMCID: PMC7060462
- DOI: 10.1111/cas.14296
Efficient recruitment of c-FLIPL to the death-inducing signaling complex leads to Fas resistance in natural killer-cell lymphoma
Abstract
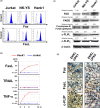
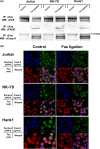
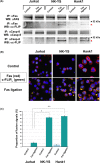
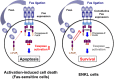
Activation-induced cell death (AICD) mediated by the Fas/Fas ligand (FasL) system plays a key role in regulating immune response. Although normal natural killer (NK) cells use this system for their homeostasis, malignant NK cells seem to disrupt the process. Extranodal NK/T-cell lymphoma, nasal type (ENKL) is a rare but fatal disease, for which novel therapeutic targets need to be identified. We confirmed that ENKL-derived NK cell lines NK-YS and Hank1, and primary lymphoma cells expressed procaspase-8/FADD-like interleukin-1β-converting enzyme (FLICE) modulator and cellular FLICE-inhibitory protein (c-FLIP), along with Fas and FasL. Compared with Fas-sensitive Jurkat cells, NK-YS and Hank1 showed resistance to Fas-mediated apoptosis in spite of the same expression levels of c-FLIP and the death-inducing signaling complex (DISC) formation. Unexpectedly, the long isoform of c-FLIP (c-FLIPL ) was coimmunoprecipitated with Fas predominantly in both ENKL-derived NK cell lines after Fas ligation. Indeed, c-FLIPL was more sufficiently recruited to the DISC in both ENKL-derived NK cell lines than in Jurkat cells after Fas ligation. Knockdown of c-FLIPL per se enhanced autonomous cell death and restored the sensitivity to Fas in both NK-YS and Hank1 cells. Although ENKL cells are primed for AICD, they constitutively express and efficiently utilize c-FLIPL , which prevents their Fas-mediated apoptosis. Our results show that c-FLIPL could be a promising therapeutic target against ENKL.
Keywords: Fas; FasL; activation-induced cell death; c-FLIPL; extranodal NK/T-cell lymphoma, nasal type.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
All authors declare no conflict of interest.
Figures


Similar articles
-
Resistance to Fas-mediated apoptosis is restored by cycloheximide through the downregulation of cellular FLIPL in NK/T-cell lymphoma.Lab Invest. 2005 Jul;85(7):874-84. doi: 10.1038/labinvest.3700291. Lab Invest. 2005. PMID: 15924153
-
Fas-associated death domain protein (FADD) and caspase-8 mediate up-regulation of c-Fos by Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) via a FLICE inhibitory protein (FLIP)-regulated pathway.J Biol Chem. 2001 Aug 31;276(35):32585-90. doi: 10.1074/jbc.M100444200. Epub 2001 May 30. J Biol Chem. 2001. PMID: 11384965
-
Regulation of the Fas death pathway by FLICE-inhibitory protein in primary human B cells.J Immunol. 2000 Sep 15;165(6):3023-30. doi: 10.4049/jimmunol.165.6.3023. J Immunol. 2000. PMID: 10975811
-
Regulation of CD95/Fas signaling at the DISC.Cell Death Differ. 2012 Jan;19(1):36-41. doi: 10.1038/cdd.2011.155. Epub 2011 Nov 11. Cell Death Differ. 2012. PMID: 22075988 Free PMC article. Review.
-
c-FLIP, a master anti-apoptotic regulator.Exp Oncol. 2012 Oct;34(3):176-84. Exp Oncol. 2012. PMID: 23070002 Free PMC article. Review.
Cited by
-
Possible Mechanisms of Lymphopenia in Severe Tuberculosis.Microorganisms. 2023 Oct 27;11(11):2640. doi: 10.3390/microorganisms11112640. Microorganisms. 2023. PMID: 38004652 Free PMC article. Review.
References
-
- Ida H, Robertson MJ, Voss S, et al. CD94 ligation induces apoptosis in a subset of IL‐2‐stimulated NK cells. J Immunol. 1997;159:2154‐2160. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

