Valproic Acid Addresses Neuroendocrine Differentiation of LNCaP Cells and Maintains Cell Survival
- PMID: 31908413
- PMCID: PMC6927225
- DOI: 10.2147/DDDT.S229930
Valproic Acid Addresses Neuroendocrine Differentiation of LNCaP Cells and Maintains Cell Survival
Abstract
Purpose: Neuroendocrine differentiation of prostate cancer, induced by androgen deprivation therapy, is mainly related to advanced disease and poor clinical outcome. Genetic and epigenetic alterations are the key elements of the prostate carcinogenesis. A group of compounds able to induce changes in this sense is inhibitors of histone deacetylase, to which it belongs valproic acid (VPA). In the present paper, we evaluated the role of this molecule on the neuroendocrine differentiation of LNCaP cells together with the effect on proliferation and survival signals.
Methods: Cell growth was analyzed by MTT and flow cytometry, while expression of proteins through Western blot analysis.
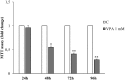
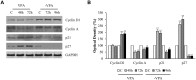
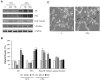
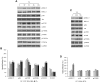
Results: Our results have documented that VPA in LNCaP cells reduces cell proliferation, decreases the S phase and Cyclin A, and up-regulates the cyclin-dependent kinase inhibitors p21waf and p27. The acquisition of androgen-independent condition is consistent with an induction of β-III Tubulin and gamma Enolase, both markers of neuroendocrine phenotype. However, all these features cease with the removal of valproate from the culture medium, demonstrating the transitory nature of the epigenetic event. The VPA treatment does not compromise the survival phosphorylated signals of Akt, ERK1/2 and mTOR/p70S6K that remain up-regulated. Consistently, there is an increase of phospho-FOXO3a, to which corresponds the decreased expression of the corresponding oncosuppressor protein.
Conclusion: Overall, our findings indicate that VPA in LNCaP prostate tumor cells, although it reduces cell proliferation, is able to drive neuroendocrine phenotype and to maintain the survival of these cells. Keeping in mind that neuroendocrine differentiation of prostate cancer appears to be associated with a poor prognosis, it is necessary to develop new treatments that do not induce neurodifferentiation but able to counteract cell survival.
Keywords: cell cycle; cell proliferation; neuroendocrine tumor; prostate cancer.
© 2019 Giordano et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Valproic acid induces neuroendocrine differentiation and UGT2B7 up-regulation in human prostate carcinoma cell line.Drug Metab Dispos. 2007 Jun;35(6):968-72. doi: 10.1124/dmd.107.014662. Epub 2007 Mar 19. Drug Metab Dispos. 2007. PMID: 17371798
-
Does valproic acid induce neuroendocrine differentiation in prostate cancer?J Biomed Biotechnol. 2011;2011:607480. doi: 10.1155/2011/607480. Epub 2010 Oct 25. J Biomed Biotechnol. 2011. PMID: 20981253 Free PMC article.
-
Low dosed interferon alpha augments the anti-tumor potential of histone deacetylase inhibition on prostate cancer cell growth and invasion.Prostate. 2012 Dec 1;72(16):1719-35. doi: 10.1002/pros.22525. Epub 2012 Apr 2. Prostate. 2012. PMID: 22473339
-
Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells.Oncologist. 2007 Aug;12(8):942-51. doi: 10.1634/theoncologist.12-8-942. Oncologist. 2007. PMID: 17766653
-
Cytotoxic Effects of Valproic Acid on Neuroendocrine Tumour Cells.Neuroendocrinology. 2016;103(5):578-91. doi: 10.1159/000441849. Epub 2015 Oct 28. Neuroendocrinology. 2016. PMID: 26505883
Cited by
-
Phytochemical Profile and In Vitro Antioxidant and Photobiological Properties of Different Extracts from Prangos ferulacea Lindl.Antioxidants (Basel). 2023 Feb 5;12(2):384. doi: 10.3390/antiox12020384. Antioxidants (Basel). 2023. PMID: 36829943 Free PMC article.
-
Potent FOXO3a Activators from Biologically Active Compound Library for Cancer Therapeutics: An in silico Approach.Appl Biochem Biotechnol. 2023 Aug;195(8):4995-5018. doi: 10.1007/s12010-023-04470-5. Epub 2023 Apr 5. Appl Biochem Biotechnol. 2023. PMID: 37017892
-
Valproic acid inhibits cell growth in both MCF-7 and MDA-MB231 cells by triggering different responses in a cell type-specific manner.J Transl Med. 2023 Mar 2;21(1):165. doi: 10.1186/s12967-023-04015-8. J Transl Med. 2023. PMID: 36864445 Free PMC article.
-
From HDAC to Voltage-Gated Ion Channels: What's Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer.Cancers (Basel). 2022 Sep 10;14(18):4401. doi: 10.3390/cancers14184401. Cancers (Basel). 2022. PMID: 36139561 Free PMC article. Review.
-
FOXO3a and Its Regulators in Prostate Cancer.Int J Mol Sci. 2021 Nov 20;22(22):12530. doi: 10.3390/ijms222212530. Int J Mol Sci. 2021. PMID: 34830408 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

