Inactivation of FOXO1 induces T follicular cell polarization and involves angioimmunoblastic T cell lymphoma
- PMID: 31908892
- PMCID: PMC6936234
- DOI: 10.20892/j.issn.2095-3941.2019.0115
Inactivation of FOXO1 induces T follicular cell polarization and involves angioimmunoblastic T cell lymphoma
Erratum in
-
Erratum to Inactivation of FOXO1 induces T follicular cell polarization and involves angioimmunoblastic T cell lymphoma.Cancer Biol Med. 2020 Feb 15;17(1):xx. doi: 10.20892/j.issn.2095-3941.2019.0374. Cancer Biol Med. 2020. PMID: 32296592 Free PMC article. No abstract available.
Abstract
Objective: Angioimmunoblastic T cell lymphoma (AITL) is an aggressive form of non-Hodgkin lymphoma derived from mature T cells. However, the underlying pathogenesis of AITL remains unresolved. We aimed to explore the role of FOXO1-mediated signaling in the tumorigenesis and progression of AITL.
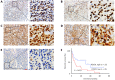
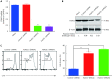
Methods: FOXO1 expression was assessed using immunohistochemistry on a total of 46 AITL tissue samples. Retroviruses encoding FOXO1 shRNA were used to knockdown FOXO1 expression in CD4+ T cells. Flow cytometric assays analyzed the proliferation and survival of FOXO1 knockdown CD4+ T cells. Furthermore, we performed adoptive T-cell transfer experiments to identify whether inactivation of FOXO1 induced neoplastic follicular-helper T (Tfh) cell polarization and function.
Results: Patients with low FOXO1 protein levels were prone to have an advanced tumor stage (P = 0.049), higher ECOG ps (P = 0.024), the presence of bone marrow invasion (P = 0.000), and higher IPI (P = 0.035). Additionally, the survival rates of patients in the FOXO1 high-expression group were significantly better than those in the FOXO1 low-expression group (χ2 = 5.346, P = 0.021). We also observed that inactivation of FOXO1 increased CD4+ T cell proliferation and altered the survival and cell-cycle progression of CD4+ T cells. Finally, we confirmed that inactivation of FOXO1 induces Tfh cell programing and function.
Conclusions: Inactivation of FOXO1 in AITL plays a key role in the tumorigenesis and progression of AITL. We propose that FOXO1 expression could be a useful prognostic marker in AITL patients to predict poor survival, and to design appropriate therapeutic strategies.
Keywords: Angioimmunoblastic T cell lymphoma; FOXO1; differentiation; inactivation.
Copyright 2019 Cancer Biology & Medicine.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
